College of Health
45 Intramuscular Macrophage Delivery Improves Aged Muscle Function After Disuse Atrophy
Elena Yee and Patrick Ferrara
Faculty Mentor: Micah Drummond (Physical Therapy & Athletic Training, University of Utah)
Abstract
Aging muscle and extended periods of disuse and/or injury leading to muscle atrophy compromises the immune system causing a dysfunctional macrophage response in muscle regrowth. This study examines the effects of injecting young adult and aged recipient mice with pro-inflammatory macrophages into the hindlimb muscles to detect if it stimulates an immune response that improves muscle recovery, after following a period of disuse atrophy. The experiment involved the recipient mice that underwent 2-weeks of hindlimb unloading muscle atrophy followed by a 4-day recovery period. Bone marrow-derived macrophages were isolated from three donor groups: young adult mice, aged mice, and 4-day reloaded aged mice. These cells were cultured in a dish, changed to pro-inflammatory macrophages, and then injected into the right hindlimb of the recipient mice after 1-day of ambulatory recovery from hindlimb unloading. On day 4 of recovery, the mice were humanly euthanized for analysis of the soleus muscle. Our results indicate that the delivery of pro-inflammatory macrophages from the three donor groups did not affect muscle mass when compared to the vehicle (Phosphate buffer saline, PBS) in young adult and aged mice; however, the delivery of the pro-inflammatory macrophages improves/rescues aged mice muscle recovery following disuse.
Introduction
Extended periods of muscle disuse and/or injury can lead to muscle atrophy within the skeletal muscles (1). Macrophages found within our immune system regulates/defends against infection/injury via processes of inflammation and phagocytosis by releasing growth factors that play a critical role in muscle regeneration and organization (2). The crosstalk between the immune system and skeletal muscle is known to be dysfunctional with aging during the recovery from disuse atrophy (3). This is concerning especially regarding the elderly population since they are more prone to increased risk of falling, developing an illness, and worsening disease complications (4). These macrophages have been identified as a key immune cell responsible for this dysfunctional crosstalk with aging (5).
The delivery of pro-inflammatory macrophages into the hindlimb muscles of young and aged mice has not been examined before. Therefore, to investigate this specific immunotherapy treatment, we induced muscle atrophy via hindlimb unloading for 14 days and administered pro-inflammatory macrophages from three different donor groups: young adult mice, aged mice, and 4-day reloaded aged mice into the hindlimbs of both young adult and aged mice 1 day following hindlimb unloading recovery. The purpose of this investigation is to determine if the delivery of these pro-inflammatory macrophages improves muscle recovery in aged mice. By performing this experiment, it could potentially lead to new treatments and exciting immunotherapies that could help skeletal muscle recovery following disuse atrophy especially regarding the elderly population.
Methods
Experimental outline
Both the young adult (3-6 months of age) and aged (23-26 months of age) C57BL/6 strain mice were divided into two experimental groups: the donor and recipient. Bone marrow-derived macrophages (BMDMs) were obtained from the three following donor groups which were used for intramuscular injections: ambulatory young adult mice, ambulatory aged mice, and aged mice that were hindlimb unloaded and reloaded for 4 days. The recipient young adult and aged mice underwent 2 weeks of hindlimb unloading followed by 4 days of reloading. One day after the HU intervention (Day 15), intramuscular injections were performed. Pro-inflammatory macrophages from the donor were delivered into the right hindlimb of young adult and aged recipient mice, while phosphate buffer saline (PBS, Vehicle) was administered into the left hindlimb. The mice continued to be ambulatory for three more days and then were euthanized. The soleus muscle was obtained for isometric force production and the muscle mass was recorded for analysis.
Bone-Marrow Derived Macrophage Isolation, Polarization, Delivery
Bone marrow-derived macrophages (BMDMs) were isolated from the three following donor groups: ambulatory young adult mice, ambulatory aged mice, and aged mice that were hindlimb unloaded and reloaded for 4 days. The mice were euthanized to obtain the femur and tibia bones where they were dissected and cleaned. A gauge needle with sterile PBS was used to flush the bones which were centrifuged and then resuspended in red blood cell lysis buffer. The cells were centrifuged again and resuspended in D10 media with macrophage-colony stimulating factor (M-CSF). These cells were plated for 4 days and were polarized on day 7 with D10 media, M-CSF, lipopolysaccharide, and interferon γ for 24 hours. After 24 hours, the cells were washed with 2x with PBS and scraped in trypsin. Cells were centrifuged and resuspended in PBS (2 million cells/100µL) that were rear-loaded into a syringe. 100µL of the polarized pro-inflammatory macrophages were administered into the right hindlimb of the recipient mice.
Ex-vivo Muscle Force Production
The soleus muscles were carefully sutured at each tendon and dissected from the mice while under isoflurane anesthesia before the measurement of muscle function. Force-frequency analyses were performed via stimulations ranging from 10-200 Hz with 1 minute between each stimulation. A dual-mode lever force transducer software (Aurora Scientific) was used to measure force and analyze data. The soleus muscle mass and length were measured and recorded.
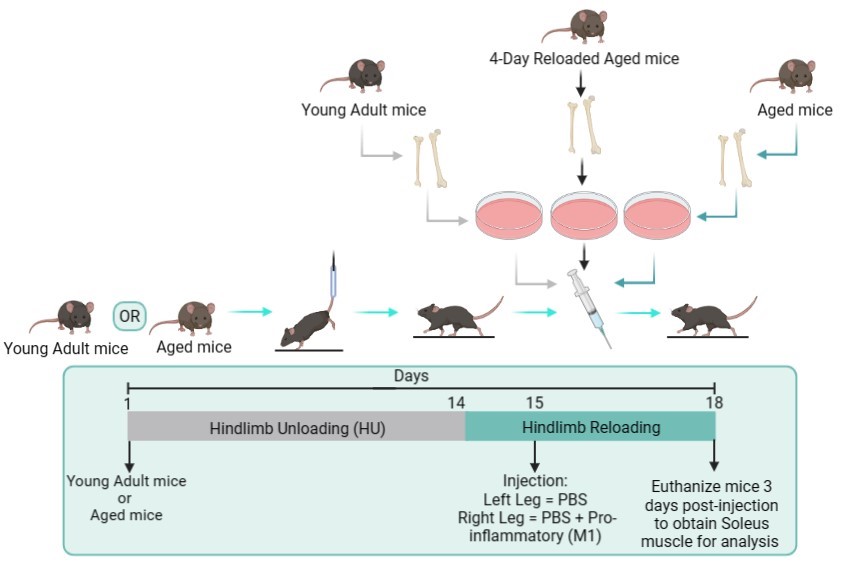
Figure 1: Schematic of experimental design.
Results
Our results indicate that the delivery from any of the three donor groups actually helped improve muscle recovery in aged mice that underwent the hindlimb unloading intervention. As indicated in figures 2-4, part D, there was an effect of treatment and the asterisk indicate that within that specific time point on the force-frequency curve, there was a significant difference between the vehicle and the aged pro-inflammatory macrophages. While, on the other hand, the young adult mice that underwent the hindlimb unloading intervention had no effect of treatment and no significant difference within the force-frequency curves (figures 2-4, part B). The bar graphs found in figures 2-4, part A and C, indicate that the muscle mass of the soleus for both the vehicle and the macrophage injections had no significant difference.
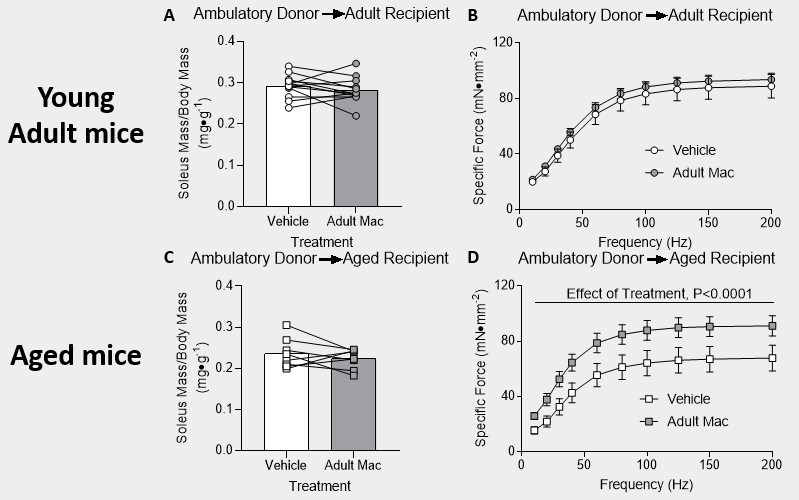
Figure 2: Delivery of young adult pro-inflammatory macrophages improved recovery in aged mice after hindlimb unloading. (A & B) Young adult mice and (C & D) aged mice were injected with PBS in the left hindlimb and young adult pro-inflammatory macrophages in the right hindlimb. (A & C) Mass of the soleus muscle relative to the body mass of the mice. P-values are based on a two-tailed t-test. (B & D) Tetanic force production during a force frequency curve. A 2-way ANOVA with sidak’s multiple comparisons test was used. Data are presented as mean ± SEM.
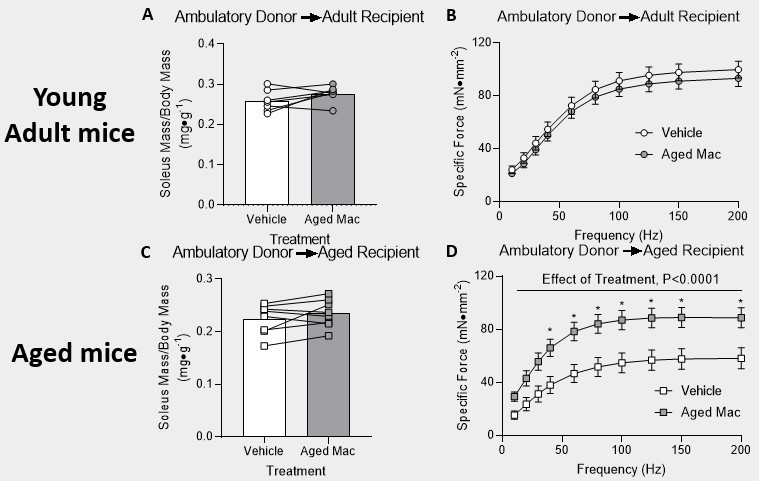
Figure 3: Delivery of aged pro-inflammatory macrophages improved recovery in aged mice after hindlimb unloading. (A & B) Young adult mice and (C & D) aged mice were injected with PBS in the left hindlimb and aged pro-inflammatory macrophages in the right hindlimb. (A & C) Mass of the soleus muscle relative to the body mass of the mice. P-values are based on a two-tailed t-test. (B & D) Tetanic force production during a force frequency curve. A 2-way ANOVA with sidak’s multiple comparisons test was used. Data are presented as mean ± SEM. *P<0.05 versus PBS.
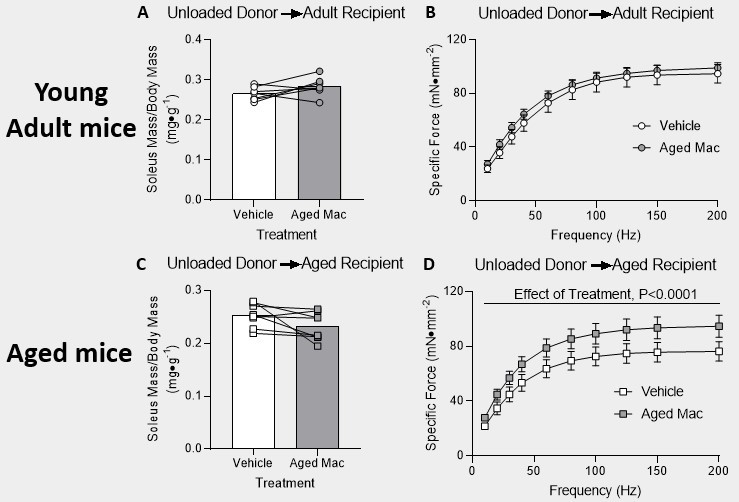
Figure 4: Delivery of pro-inflammatory macrophages from 4-day reloaded aged mice improved recovery in aged mice after hindlimb unloading. (A & B) Young adult mice and (C & D) aged mice were injected with PBS in the left hindlimb, and aged mice reloaded for 4 days following 14 days of HU (unloaded) pro-inflammatory macrophages in the right hindlimb. (A & C) Mass of the soleus muscle relative to the body mass of the mice. P-values are based on a two-tailed t-test. (B & D) Tetanic force production during a force frequency curve. A 2-way ANOVA with sidak’s multiple comparisons test was used. Data are presented as mean ± SEM.
Discussion/Conclusion
In summary, the delivery of pro-inflammatory macrophages via intramuscular injection from young adult mice, ambulatory aged mice, and aged mice reloaded for 4 days following 14 days of hindlimb unloading helps rescue aged mice muscle recovery following disuse atrophy. Therefore, the delivery of macrophages to the soleus muscle improves muscle recovery in aged mice. These findings allow for new and exciting immunotherapies to be implemented in future studies. Two potential follow-up studies could consist of understanding/determining the mechanisms that helped improve the delivery of pro-inflammatory macrophages to aged mice and if the delivery of other macrophages, other than pro-inflammatory, can also help improve muscle recovery in aged mice. These further investigations would help to solidify our findings and understanding of the role of macrophages to help aid in muscle recovery after disuse atrophy. Page Break
References
- Nunes, Everson A et al. “Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: mechanisms, prevention, and recovery strategies.” American journal of physiology. Cell physiology vol. 322,6 (2022): C1068-C1084.
- Hammers, David W et al. “Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion.” Journal of applied physiology (Bethesda, Md. : 1985) vol.118,8 (2015).
- Wang, Ying et al. “Aging of the immune system causes reductions in muscle stem cell populations, promotes their shift to a fibrogenic phenotype, and modulates sarcopenia.” FASEB journal: official publication of the Federation of American Societies for Experimental Biology vol. 33,1 (2019): 1415-1427.
- Fried, L P et al. “Frailty in older adults: evidence for a phenotype.” The journals of gerontology. Series A, Biological sciences and medical sciences vol. 56,3 (2001): M146-56.
- Fix, Dennis K et al. “Disrupted macrophage metabolic reprogramming in aged soleus muscle during early recovery following disuse atrophy.” Aging cell vol. 20,9 (2021): e13448.

