Science
99 Exploring the Impact of Medicines on DNA Repair Enzymes
Mary Fairbanks and Martin Horvath (School Of Biological Sciences)
Faculty Mentor: Martin Horvath (School of Biological Sciences, University of Utah)
Abstract
Medicines are known to alter the gut’s microbiome composition and function, but their ability to also impact DNA repair enzymes has not yet been investigated. In this study, we added medicines into bacterial systems to see how mutation rate and DNA repair enzymes were impacted. We found that aspirin was toxic to bacteria and determined a tolerable dose through bacterial growth rate tests. A concentration of 0.01 mg/mL of aspirin showed the same replication rate as when no medicine was added, so we kept the dose below this threshold to maintain a level playing field for the following mutation suppression assay. The mutation frequency rates of bacteria exposed with various concentrations of medicines were measured through counting rifampicin resistant mutants that spontaneously arose in an overnight culture. The median mutation rate for aspirin was 22 per 100 million cells plated (14-29, 95% confidence interval; CI), which was not significantly different from the rate measured for water at 23 (19-28, 95% CI). Similarly, the median mutation rate for adapalene was 19.5 (16-28, 95% CI), which was not significantly different from the rate measured for DMSO at 24.5 (21-33, 95% CI). These results show that aspirin and adapalene do not impact mutation rates in our laboratory strain of bacteria. Previous studies suggest that nonsteroidal anti-inflammatory drugs (NSAIDs) increase the likelihood of infection from Clostridioides difficile by diminishing microbiome diversity and resistance, which raises concerns about medicines negatively impacting the microbiome. Our results reassure us that medicines like aspirin and adapalene probably do not have off-target impacts on DNA repair enzymes, and thus are unlikely to disrupt the mutation rate of bacteria in our microbiome.
Introduction
Commonly used medicines treat specific symptoms or illnesses but may have unintended side effects. Aspirin is taken to reduce pain and fever but is sometimes used to prevent heart attacks and ischemic strokes (strokes that occur when the flow of blood to the brain is blocked) (Brazier 2020). Aspirin belongs to a group of medicines called salicylates, which stop the production of certain natural substances that cause fever, pain, swelling, and blood clots. A rare side effect of daily low-dose aspirin is hemorrhagic stroke (Brazier 2020). Other side effects of aspirin include irritation of the stomach lining and digestive problems (Zimlich 2022). Variations in drug outcomes encourage us to investigate pathways at a molecular level in order to improve drug-targeting efficiency, reduce side effects, and uncover hidden uses. Drug disposition (absorption, distribution, metabolism, and excretion) and pharmacokinetics (the rate of these processes and concentration compared to time) play a vital role in drug efficacy and outcome (Caldwell, Gardner, and Swales 1995). Thus, drug interactions should be investigated in different environments in our body. Experimenting with medicines’ impact on bacteria is particularly important to depict environments like our microbiota with a non-invasive model system.
The relationship between medicines and bacteria in the gut microbiome is complex and bi-directional (Reviewed in Weersma, Zhernakova, and Fu 2020). Changes in the gut microbiome composition can be influenced by drugs, and likewise, the gut microbiome can influence drug activity by altering bioavailability of the drug. Gut microbes can impact the efficiency of a drug by transforming its structure which may change drug bioavailability, activity, or toxicity, now referred to as pharmacomicrobiomics (Koppel, Rekdal, and Balskus 2018). For example, the oral antiviral drug brivudine can be metabolized to bromovinyluracil by five major phyla that dominate the mammalian gut microbiota (Reviewed in Zimmermann et al. 2019).
Microbiome composition influences drugs and vice versa. Recent findings show commonly used non-antibiotic drugs such as proton pump inhibitors (PPIs) and metformin change the microbiome composition, biodiversity, and function (Freedberg et al. 2015). Additionally, the use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with enhanced susceptibility to Clostridioides difficile infection (CDI) with increased severity by decreasing colonization resistance and biodiversity (Bonder et al. 2016). CDI is a major public health threat that can range from diarrhea to life threatening damage to the colon. NSAIDs inhibit the activity of cyclooxygenase (COX) and lead to the formation of prostaglandins (PG), which alter the inflammatory response pathway. In a recent study, the NSAID indomethacin was found to dramatically increase CDI severity through inhibition of intestinal tissue immune response (Maseda et al. 2019). Indomethacin inhibited PG biosynthesis, which increased intestinal tissue histopathology due to dismantled intestinal epithelial tight junctions allowing partial bacterial diffusion (Maseda et al. 2019). The microbiome’s composition being impacted by medicine and its ability to respond to that medicine shows an example of the interaction between drugs and gut microbiome to be both complicated and interconnected.
Moreover, medicines intended to treat cancer may have off-target molecular interactions that affect DNA repair function. Chronic intake of the NSAID aspirin was shown to reduce the risk of colorectal cancer (Gwyn and Sinicrope 2002). The GO DNA repair pathway is known to be involved in cancer reduction (Curtin 2012). A member of the GO DNA repair pathway, the DNA glycosylase MUTY, initiates the repair process at lesions where an oxidized purine 8-oxo-7, 8-dihydroguanine (8-oxoG) is mispaired with an adenine (A). DNA repair enzymes are responsible for preventing mutations that lead to cancerous growth in humans, so aspirin may reduce the risk of colon cancer by impacting the function of DNA repair enzymes. Variants of the mammalian homolog MUTYH are known to increase the risk of cancer (Picanço-Albuquerque et al. 2022). To explore the idea that aspirin and other medicines can interact with MUTYH, medicines were docked to MUTYH protein with favorable binding energies (Sehgal 2021). Additionally, MutY glycosylase assays performed by Harini Srinivasan and Sonia Sehgal found adapalene to reduce the activity of bacterial MutY (Srinivasan 2022). The finding that adapalene inhibits MutY suggests that it may also inhibit human MUTYH which could be linked to a higher risk for cancer.
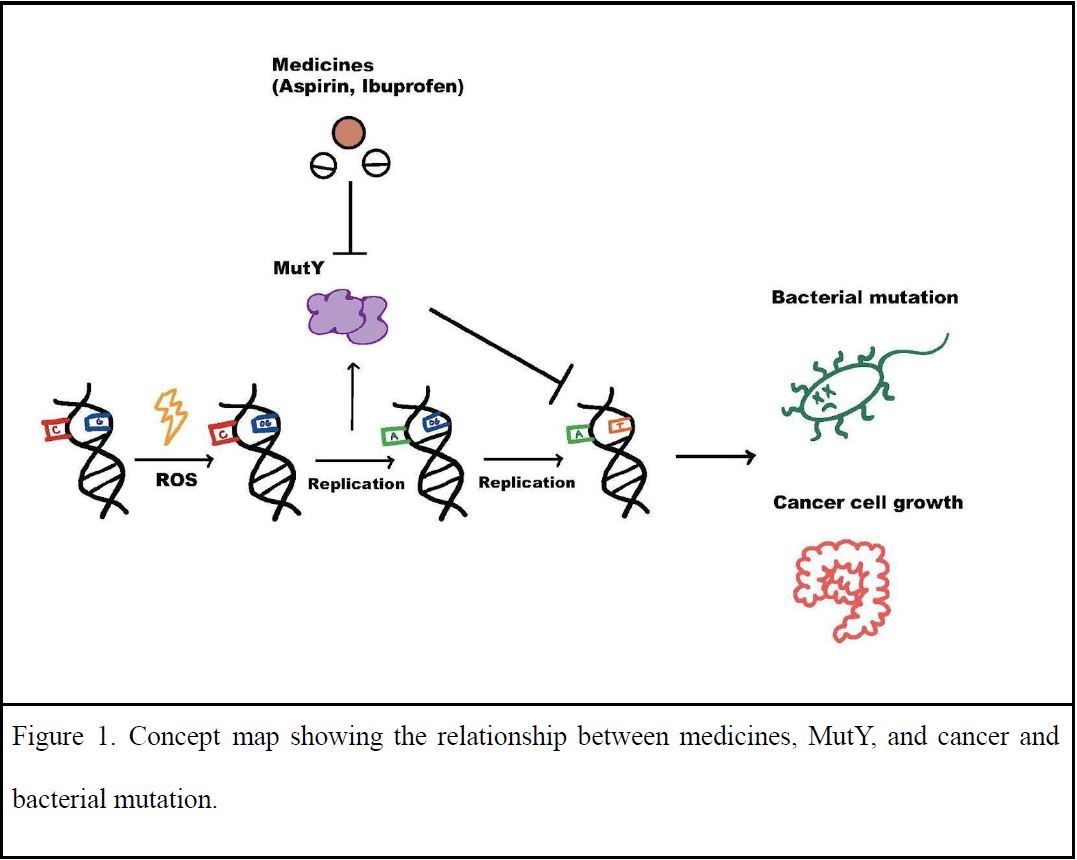
To guide our research, we posed the following questions: do these medicines have the potential for treating or preventing colorectal cancer because they act on DNA repair enzymes? Are the DNA repair enzymes in our cells and our gut microbiota being impacted when we take these medicines? To answer these questions, we added medicines into bacterial systems to see how bacterial mutation rate and DNA repair enzymes were affected (Figure 1). Mutations rates were measured in the presence of medicines to evaluate MutY function. Since mutation rate and replication rate are related, replication rate with and without the medicine was also measured to set a baseline for the mutation rate assay. Noticeable differences in bacterial growth rates were observed when adding medicines like aspirin, but are unlikely due to impacting the function of DNA repair enzymes like MutY as no impact on the mutation rate was detected.
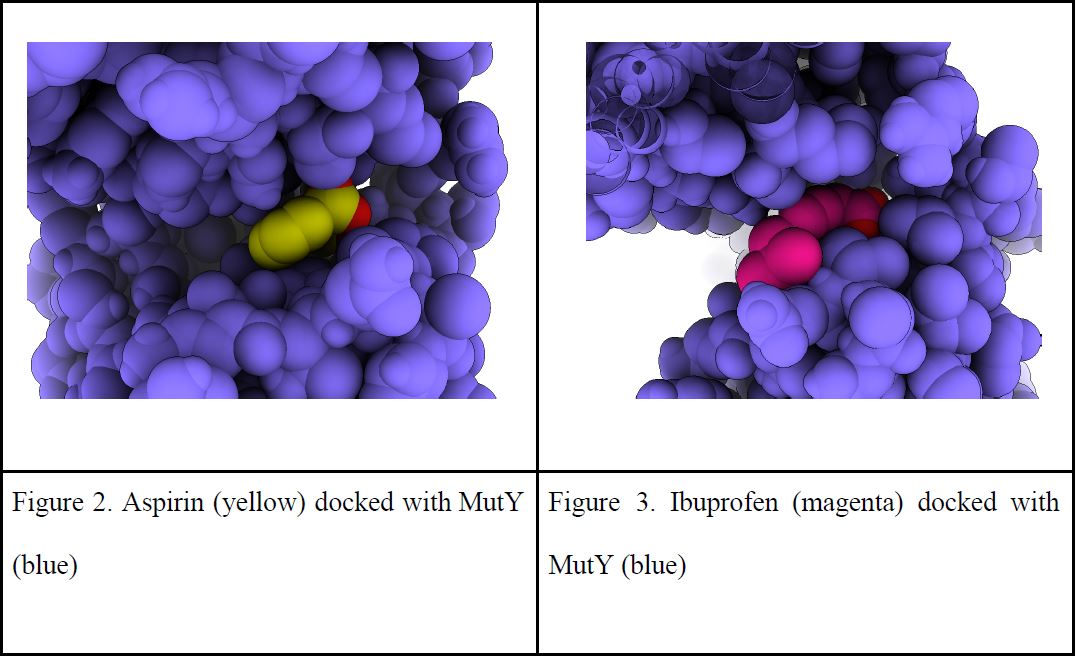
| Figure 2. Aspirin (yellow) docked with MutY (blue) | Figure 3. Ibuprofen (magenta) docked with MutY (blue) |
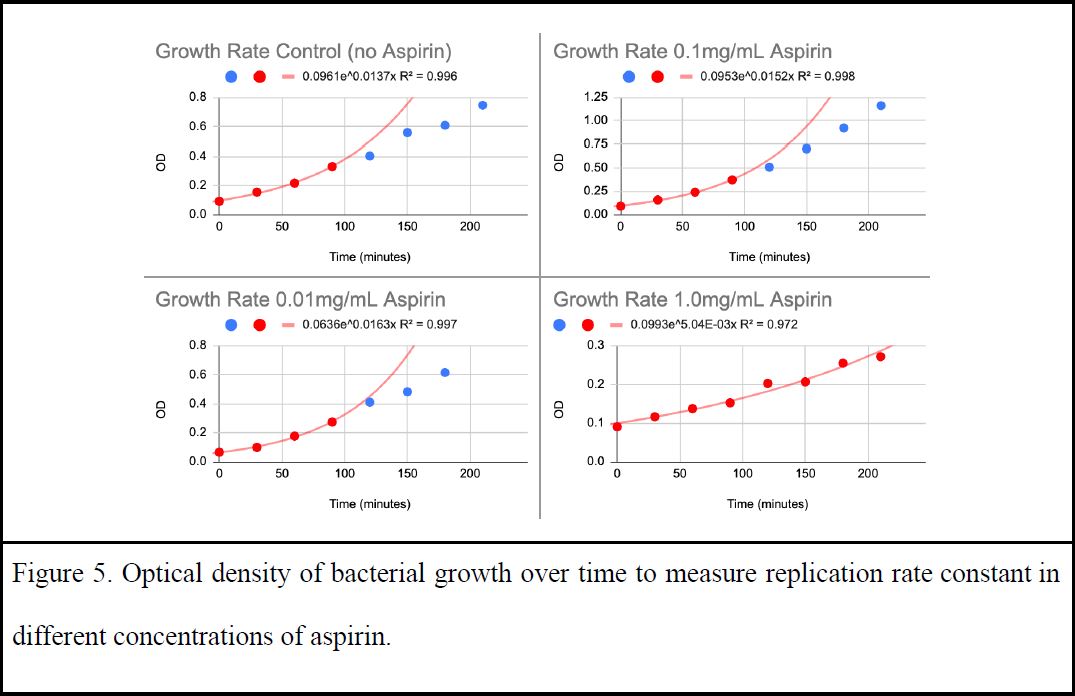
Bacteria expressing MutY were grown in the presence of these medicines. Initial trials tested 10mg/mL of aspirin added to 2mL overnight culture of KAT media. Culture tubes with aspirin had no growth compared to those that had no medicine. Medicines were then spotted to media plates using a Kirby Beuer technique, this time including ibuprofen. There was a clear zone of bacterial inhibition when aspirin was added to the plates as opposed to ibuprofen which had no zone of inhibition (Figure 4). Toxicity of aspirin was then examined by viewing relative sizes of zones of inhibition in various dilutions of medicine to verify a safe, non-toxic level (Figure 4). Since MutY works on DNA damage before replication, the growth rate of bacteria with various concentrations of medicine was measured to accurately represent mutation occurrences in the proceeding assay. A replication rate across the tested types of medicine was also needed to create an equal level playing ground through the adjustment of concentrations. Growth of bacteria populations was monitored by optical density over time. This growth test was repeated multiple times to plot 2-3 trials for each concentration of aspirin in order to examine which concentration had similar replication rates to the control (no medicine) (Figure 5). Aspirin was found to be tolerated at a low concentration of 0.01mg/mL while ibuprofen, DMSO, and water had no impact on growth rate.

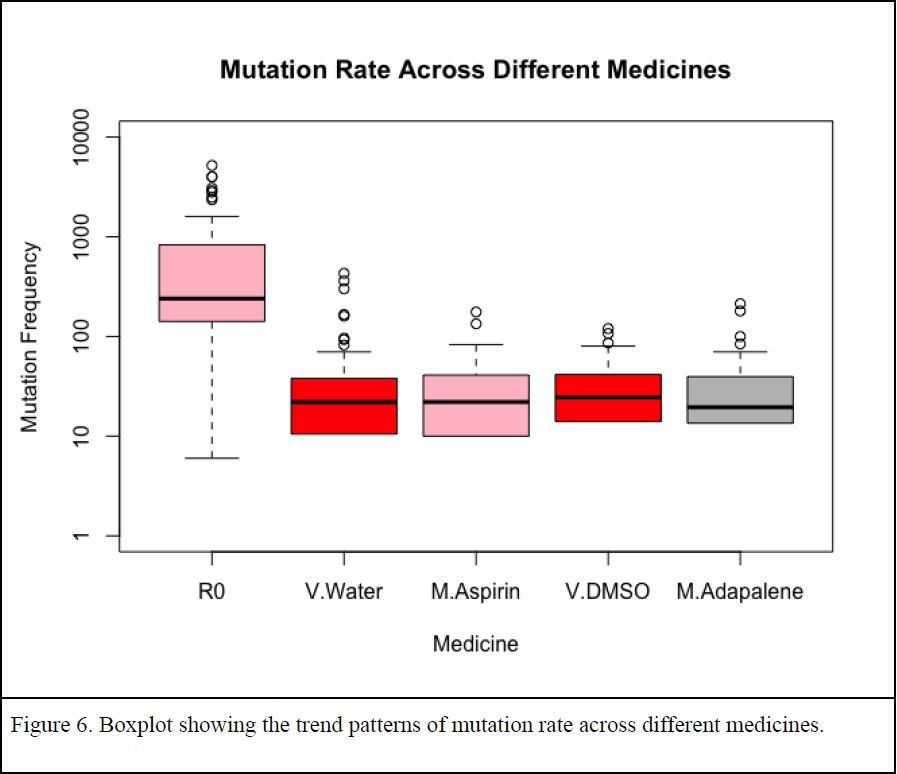
To measure mutation rates, several replica cultures were grown with and without medicine by students in the Molecular Biology of DNA Lab course (BIOL 3510, Fall 2022). Spontaneous mutations arising in these cultures were detected by acquired ability to grow on plates containing the antibiotic rifampicin. The boxplot created by the data from members of the DNA Lab Fall 2022 showed a far higher mutation frequency for cultures that do not express MutY (null) compared to cultures that express a functional MutY (Figure 6). This pattern indicates that the mutation rate assay is working as intended, as a higher mutation rate is expected if DNA repair enzymes are missing or not functioning properly. The mutation frequency measured for cultures expressing MutY and grown in the presence of medicine was highly comparable to that measured in the absence of added medicines (Figure 6).
The statistical significance of these comparisons was evaluated with a bootstrap method to provide confidence intervals. Overlapping confidence intervals indicate the data are highly comparable, whereas non-overlapping confidence intervals indicate the data are noticeably different at the 95% confidence level (p < 0.05). As reported in Table 1, null and MutY expressing cultures differ significantly in the measured mutation frequency as indicated by non-overlapping confidence intervals. By contrast, adding medicine to the cultures did not impact mutation rates as indicated by overlapping confidence intervals for control cultures grown with added vehicles and cultures grown in the presence of medicines (Table 1).
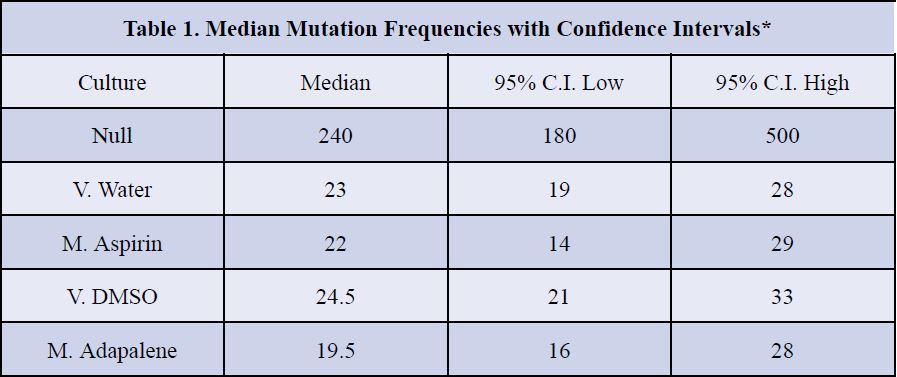
*Confidence intervals were calculated by a bootstrap method and show statistical significance by nonoverlap.
Discussion
Understanding how medicines impact the function of MutY will not only be applicable to knowing its impact on cancer, but also the mutation rate of enzymes in our microbiota. Learning how these medicines impact bacterial enzymatic function will teach us ways to increase drug efficiency and improve various aspects of our health. Biological tests were put on hold as COVID 19 shut down in person lab work. We stepped back from lab work to focus on designing experiments with as much information as we could get from online sources. Previous experiments conducted by Sonia Seghal and Harini Srinivasan (former Horvath lab members) showed the glycosylase activity of MutY to be influenced by medicines, especially adapalene which normally treats acne. These findings encouraged us to dock other medicines to DNA repair enzymes with molecular modeling tools/programs to see if binding affinity was favorable. To explore the role that medicines have on DNA repair enzymes, medicines were docked to a crystal structure of MutY using a virtual protein modeling software program and docked using AutoDock VINA. This program calculated favorable binding energies within MutY and its OG:A substrates (oxidized guanine:adenine). Aspirin, ibuprofen, adapalene, and other medicines were also shown to favorably bind to sites in the N terminal domain where adenine interacts with catalytic residues and in the C terminal domain which recognizes the damaged base OG. These favorable binding interactions added support to the idea that medicines have the potential to alter the function of DNA repair enzymes. When we were able to return to lab work, we measured mutation rates by counting mutants that spontaneously arose in overnight cultures of bacteria exposed with various concentrations of medicines. To our surprise, adding aspirin killed overnight cultures. Toxicity to aspirin was a major impediment to testing that idea that aspirin impacts DNA repair in living cells as dead cells cannot mutate. We reaffirmed the concentrations medicines have an effect on growth or replication rate through toxicity tests and bacterial population growth curves. A tolerable dose was determined through comparing growth rates of bacteria cultures in media with varying concentrations of aspirin in comparison to control cultures which were grown in media with no added aspirin. A concentration of 0.01 mg/mL of aspirin showed the same replication rate as when no medicine was added, so we kept the dose below this threshold to maintain a level playing field between the tested medicines. With concentrations adjusted, the median mutation rate for aspirin and adapalene was found to not significantly differ from the solvents water and DMSO. These results show that aspirin and adapalene do not impact mutation rates in our laboratory strain of bacteria. Previous studies suggest that NSAIDs like aspirin increase the likelihood of infection from Clostridioides difficile by diminishing microbiome diversity and resistance, which raises concerns about medicines negatively impacting the microbiome (Maseda et al. 2019). However, our results show that medicines like aspirin and adapalene probably do not have off-target impacts on DNA repair enzymes, and thus are unlikely to disrupt the mutation rate of bacteria in our microbiome.
The molecular modeling and biochemistry results have varied findings on the impact of medicines on DNA repair function. We thought that the medicines may act as competitive inhibitors and therefore reduce enzymatic function. When medicines were docked, we saw binding of both aspirin and ibuprofen to the active site and predicted medicines would impact function. Through findings from a biochemical glycosylase assay, adapalene was seen to reduce the function of bacterial MutY in an isolated system (only protein and substrate). Reduction in function of the MutY enzyme could be explained by competitive inhibition from adapalene binding to the active site. However, the function of MutY in a biological system appeared to not be impacted as mutation rates measured for bacterial cultures were relatively unchanged by medicines. The differing results seen in a biochemical and biological setting may be due to drug bioavailability, which refers to the extent and rate that a drug is absorbed into a living system. If the medicine cannot get into the bacterial cells or if the medicine is being metabolized by the bacteria then the opportunity for interacting with the DNA repair enzyme would be lost. While the biochemical and biological approaches had varying results, information from both systems can help us uncover mechanisms involving drug pathways.
The tested medicines were not seen to have an impact on bacterial mutation rates through use of DNA repair enzymes like MutY. However, these findings can help fine-tune pathways to increase drug efficiency and explore unknown roles about other similar protein-substrate interactions. For example, a eukaryotic homolog to the enzyme in this experiment is MUTYH, which when dysfunctional may lead to the inheritable MUTYH-associated polyposis (MAP) variants linked to colorectal cancer in humans (Nakamura et al. 2021). This homolog interests us as we would like to see its impact in medical settings. Understanding the relationship between commonly taken medications and their corresponding enzymatic pathways might inspire ways to improve cancer treatments. Medications that cause inhibition of DNA repair enzymes such as MUTYH may enhance the use of current therapies such as chemotherapy and radiation therapy, which induce DNA damage. Alternatively, increased function in a particular repair pathway could also be used as a monotherapy by selectively killing cancer cells.
Future Directions
Through previous studies of cell survival with some of our tested medicines, aspirin was thought to promote apoptosis through inducing an endoplasmic reticulum (ER) stress response (Ausina et al. 2020). The inhibited growth seen in aspirin could be explained by salicylic acid damaging the cell membrane, but does not account for if salicylic acid induces an ER stress response, as ER is only present in eukaryotes. In future experiments, yeast should be tested to see if aspirin makes a more noticeable difference in cell survival or mutation rates. Switching to a eukaryotic model system will help explain the mechanisms involved in both our cells’ and our microbiota’s DNA repair enzymes when taking commonly used medications.
Methods
To examine the impact of FDA approved medicines on MutY, mutation rates of bacterial cultures with and without medicines were tested. A plasmid encoding MutY was transformed into the CC104 reporter strain that otherwise does not express MutY or MutM. Null cultures with no functioning MutY and other cultures containing functioning MutY genes were grown overnight. Some of the cultures with MutY were treated with medicine and the other cultures were treated with the vehicle water or dimethyl sulfoxide (DMSO). Culture media additionally contained 10 µg/mL kanamycin, 100 µg/mL ampicillin and 12.5 µg/mL tetracycline. Cultures were grown for 18 hours at 37 degrees with shaking at 180 rpm. Cells were harvested by centrifugation and washed in 0.85% sodium chloride before seeding the equivalent of 100 µL culture on rifampicin plates to measure the frequency of rifampicin resistant spontaneous mutants. Washed cells were additionally diluted 1:107 and seeded on media plates without rifampin to measure cell density. These values were analyzed and plotted with R.
Acknowledgments
This research was made possible by the students of the Fall 2022 and Fall 2023 DNA lab course taught at the University of Utah, Quyen Tran, Maggie Leavitt, Vincent Mays, and the members of the Horvath research team. This research was also funded by the National Science Foundation (NSF) award number 1905249 under Dr. Horvath.
ReferencesAusina, Priscila, Jessica R. Branco, Thainá M. Demaria, Amanda M. Esteves, João Gabriel B. Leandro, Alan C. Ochioni, Ana Paula M. Mendonça, et al. 2020. “Acetylsalicylic Acid and Salicylic Acid Present Anticancer Properties against Melanoma by Promoting Nitric Oxide-Dependent Endoplasmic Reticulum Stress and Apoptosis.” Scientific Reports 10
(1): 19617. https://doi.org/10.1038/s41598-020-76824-6.
Bonder, Marc Jan, Ettje F. Tigchelaar, Xianghang Cai, Gosia Trynka, Maria C. Cenit, Barbara Hrdlickova, Huanzi Zhong, et al. 2016. “The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome.” Genome Medicine 8 (April): 45. https://doi.org/10.1186/s13073-016-0295-y.
Brazier, Yvette. 2020. “Aspirin: Health Benefits, Uses, Risks, and Side Effects.” September 11, 2020. https://www.medicalnewstoday.com/articles/161255.
Caldwell, J., I. Gardner, and N. Swales. 1995. “An Introduction to Drug Disposition: The Basic Principles of Absorption, Distribution, Metabolism, and Excretion.” Toxicologic Pathology 23 (2): 102–14. https://doi.org/10.1177/019262339502300202.
Curtin, Nicola J. 2012. “DNA Repair Dysregulation from Cancer Driver to Therapeutic Target.”Nature Reviews Cancer 12 (12): 801–17. https://doi.org/10.1038/nrc3399.
Freedberg, Daniel E., Nora C. Toussaint, Sway P. Chen, Adam J. Ratner, Susan Whittier, Timothy C. Wang, Harris H. Wang, and Julian A. Abrams. 2015. “Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial.” Gastroenterology 149 (4): 883-885.e9. https://doi.org/10.1053/j.gastro.2015.06.043.
Gwyn, Karin, and Frank A Sinicrope. 2002. “Chemoprevention of Colorectal Cancer.” The American Journal of Gastroenterology 97 (1): 13–21. https://doi.org/10.1016/S0002-9270(01)03997-1.
Koppel, Nitzan, Vayu Maini Rekdal, and Emily P. Balskus. 2018. “Chemical Transformation of Xenobiotics by the Human Gut Microbiota.” Science (New York, N.y.) 356 (6344): eaag2770. https://doi.org/10.1126/science.aag2770.
Maseda, Damian, Joseph P. Zackular, Bruno Trindade, Leslie Kirk, Jennifer Lising Roxas, Lisa
M. Rogers, Mary K. Washington, et al. 2019. “Nonsteroidal Anti-Inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium Difficile Colitis While Dysregulating the Inflammatory Response.” MBio 10 (1): e02282-18. https://doi.org/10.1128/mBio.02282-18.
Picanço-Albuquerque, Clarissa Gondim, Rosane Oliveira Sant’Ana, Maria Claudia dos Santos Luciano, Maria Júlia Barbosa Bezerra, Flávio da Silveira Bitencourt, Francisca Fernanda Barbosa Oliveira, Thuany Pinto Rocha de Souza, Paulo Goberlânio de Barros Silva, Marcos Venício Alves Lima, and Isabelle Joyce de Lima Silva-Fernandes. 2022. “MUTYH-Related Polyposis Syndrome Associated with a Variant of Uncertain Significance Mutation: A New Pathogenic Mutation?” Journal of Clinical Oncology 40 (16_suppl): e22528–e22528. https://doi.org/10.1200/JCO.2022.40.16_suppl.e22528.
Sehgal, Sonia. 2021. “EXPLORING THE ROLE OF BIOLOGICAL PROBES IN DNA REPAIR ENZYME MUTYH.” https://doi.org/2021.
Srinivasan, Harini. 2022. “Srinivasan_Thesis.Pdf.” https://drive.google.com/file/d/1TvQThafeo_HqHiRkoHruWwBIEeeJwr4h/view?usp=e mbed_facebook.
UCSF Chimera–a visualization system for exploratory research and analysis. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. J Comput Chem. 2004 Oct;25(13):1605-12.
Weersma, Rinse K., Alexandra Zhernakova, and Jingyuan Fu. 2020. “Interaction between Drugs and the Gut Microbiome.” Gut 69 (8): 1510–19. https://doi.org/10.1136/gutjnl-2019-320204.
Zimlich, Rachael. 2022. “Aspirin vs. Ibuprofen: How Are They Different, When to Use Each.” Healthline. February 2, 2022. https://www.healthline.com/health/aspirin-vs-ibuprofen.
Zimmermann, Michael, Maria Zimmermann-Kogadeeva, Rebekka Wegmann, and Andrew L. Goodman. 2019. “Separating Host and Microbiome Contributions to Drug Pharmacokinetics and Toxicity.” Science (New York, N.Y.) 363 (6427): eaat9931. https://doi.org/10.1126/science.aat9931.

