School of Medicine
68 Defining the role of CNS CD8 T cells in demyelinating disease pathogenisis
Anne Eliza P. Pugmire; Brian Evavold (Pathology); and Hiran Thyagarajan (Pathology)
Faculty Mentor: Brian Evavold (Pathology, University of Utah)
Overview:
Multiple Sclerosis (MS) is a chronic autoimmune demyelinating disease of the central nervous system (CNS), affecting ~2.8 million people worldwide (Walton et al). It is characterized by numbness, paralysis and fatigue due to the body’s immune system attacking its own CNS, with three times higher disease incidence in women. CD4 T cells have been shown to drive the inflammation against the myelin sheath in the CNS; however, other cell types including CD8 T cells are key contributors to MS pathophysiology. The experimental autoimmune encephalomyelitis (EAE) mouse model for MS involves the use of Myelin Oligodendrocyte Glycoprotein (MOG) 35-55 peptide alongside an adjuvant to monitor for disease symptoms including progressive paralysis of the hindlimbs and forelimbs of mice at defined time points. The stages include disease onset (d12-15), followed by peak paralysis (d20-25), which then progresses to a chronic disease stage (d35-100). Our data has identified distinct CD8 T cell subsets which contribute differently to disease kinetics. We observed that the differentiation of CD8 T cell subsets based on CD8α and CD8β protein expression shows a hint of variation in disease progression. The subsets have differential expression of gene targets as confirmed by RNA seq analysis and protein expression by flow cytometry. Specifically, the CD8αα cells show a regulatory expression for Ly49, central memory phenotype and downregulation of exhaustion markers.
Significance:
While much research has been done on conventional CD8 T cells in the CNS which express an alpha-beta heterodimer in the context of MS and EAE, there has been little investigation into the role of non-conventional CD8 cells that express an alpha homodimer (CD8αα cells). These cells could play a crucial role in disease pathogenesis, as they have been identified to have a regulatory phenotype in other tissues (Konkel et al, 2011).
Hypothesis:
While EAE is classically defined as a CD4 T cell mediated response, the importance of CD8 T cells in promoting demyelination cannot be overruled. Mice with the CD8 receptor gene knockout exhibit reduced disease severity. Moreover, CD8 T cells have been shown to have sustained inflammation in the presence of CD4 T cells. Thus, we hypothesize:
- CD8αα and CD8αβ subsets expand differentially and have unique contributions throughout the course of EAE progression
- CD8αα take on a regulatory phenotype
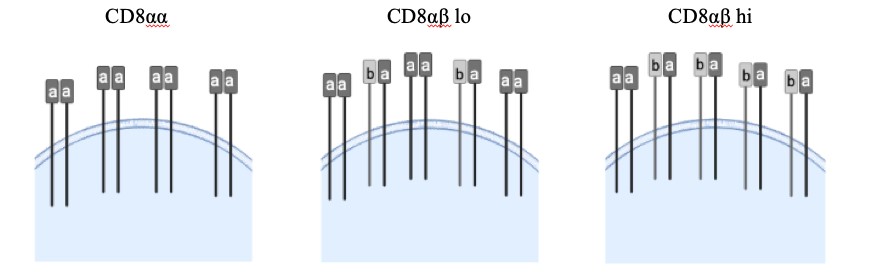
Figure 1: Each CD8 T cell has a variety of CD8 coreceptors, some of which are composed of one α and one β chain while others have two α chains. CD8αβ is the conventional CD8 T cell coreceptor that is thymus selected. However, we have also observed that there are varying levels of CD8 αα cells, which are exclusively composed of the CD8α coreceptor. Varied or mixed expression of αα and αβ coreceptors could regulate demyelinating disease outcome.
Results:
To better understand the role of CD8αα cells in the EAE model, we chose to focus on two timepoints, peak and late chronic. Here, we examined a correlation between disease severity with distribution, function and gene expression of different CD8 T cell subsets during demyelinating disease progression. While the total number of CD8 T cells remained the same across all timepoints and severity (Fig 2A), we observed a significant increase in bulk CD8 percentages for mice at late chronic stage than for mice at peak EAE (Fig 2B). Total lymphocyte cell count was increased in late chronic mice, although the percentage of total T cells was not significantly different between the two stages (Fig 2C and 2D).
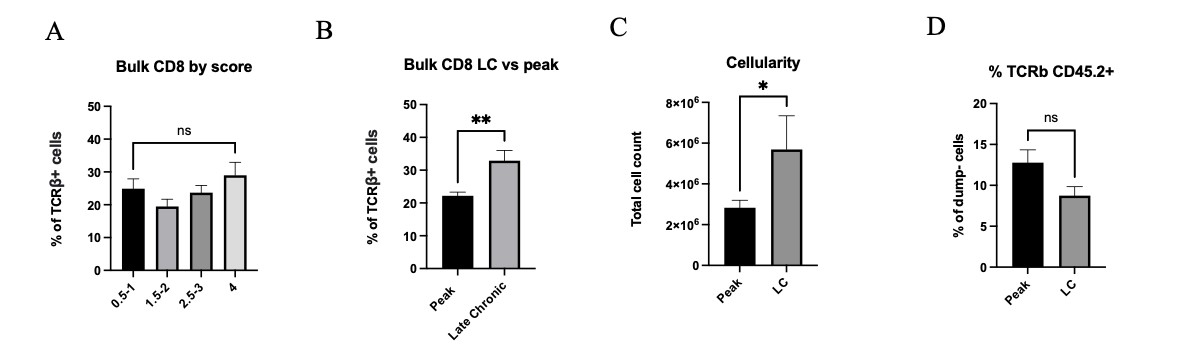
Figure 2: A) Bulk CD8 percentages across varying scores in CNS tissue. B) Bulk CD8 percentages compared between peak and late chronic timepoints. C) Total cellularity for peak vs late chronic (LC) mice. D) Percentage of TCRβ+ CD45.2+ cells for peak vs late chronic mice. Means + SEM from >3 experiments, with n=11 mice at peak and n=7 mice at late-chronic stage. ns= not significant, *p<0.1,**p<0.01.
Furthermore, through flow cytometric analysis of CNS CD8 T cells, we observed three subpopulations, CD8αα, CD8αβ low, and CD8αβ high. Mice without EAE induction The percentages of the CD8αα subset is higher in sick EAE mice than those where there was no disease induction (Fig 3A), indicating a functional role in demyelination. Additionally, when compared between the spleen and the CNS, of both sick and control mice, the CD8αα percentages in the spleen were negligible (data not shown). In contrast to bulk CD8 cells, CD8αα subset percentages correlated with score but not with the stage of disease (Fig 3B&C). At the late chronic timepoint, percentages were separated and analyzed based on severity, and we observed a significant increase in CD8αα cells in mice with a low score (Fig 3D). This increase in CD8αα cells in mice that have recovered to a lower disease severity score after a long disease course suggests a potential regulatory role of these cells in EAE disease progression.
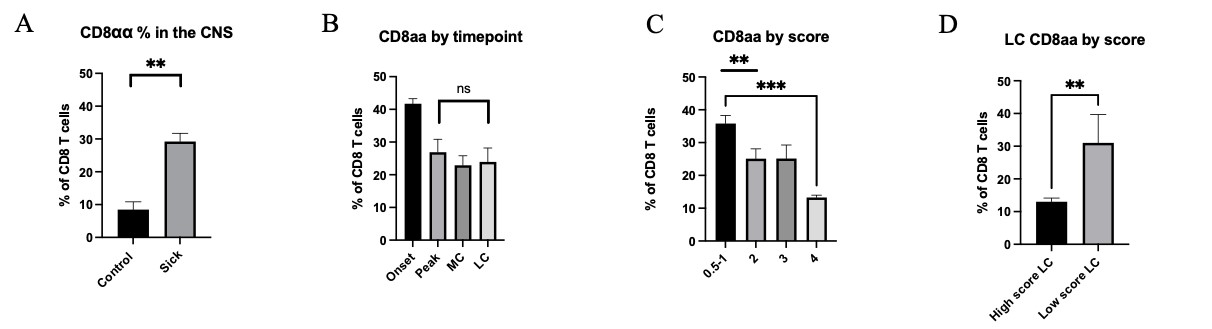
Figure 3: A) Percentage of CD8αα cells compared between non sick and sick EAE mice compiled from all timepoints and with various scores. B) Percentage of CD8αα across timepoints. C) Percentage of CD8αα across scores. D) Percentage of CD8αα in LC compared between low scores (1) and high scores (3-4). Means + SEM from >3 experiments, with n=5 mice at onset, n=10 at peak, n=3 at mid chronic and n= 10 mice at late-chronic stage. ns= not significant, *p<0.1, **p<0.01, ***p<.001
We also examined the expression pattern of various key proteins across the three different CD8 subsets. These candidates were chosen based on results from single cell RNA sequencing data that were obtained from mice at peak and chronic EAE and from studies that had reported increased expression in CD8αα IELs. Expression of KLRK (NKG2D), Ly6C, CD122, NK1.1 and Ly49C was significantly greater in CD8αα cells than in the two CD8αβ expressing subsets (Fig 4A). Expression of CD44 and CD62L was also highest in the CD8αα population, suggesting that this subset takes on a central memory phenotype (Fig 4B).
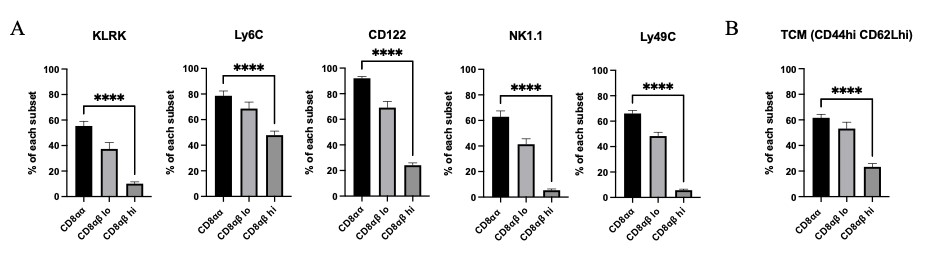
Figure 4: A) KLRK, Ly6C, CD122, NK1.1 and Ly49C expression for the three CD8 T cell subsets were analyzed from mice at peak and late-chronic EAE stages of varied scores. B) T central memory markers were analyzed from mice at peak and late-chronic EAE stages of varied scores. Means + SEM from >3 experiments, with n=10 mice. ****p<0.0001
Conclusion:
While we attempt to understand the role and function of the CD8αα cells in CNS during demyelination, our preliminary data suggests a correlation between this cell population with disease severity. Further investigation is necessary to elucidate the source of this CD8αα population (i.e. is it a downregulation of CD8β receptor on traditional CD8 T cells that gives rise to this population or do they migrate in from other areas of the body following disease induction?). In order to do this, we have ongoing experiments measuring the affinity of the TCR of CD8αα cells for peptide MHC to see how it compares to traditional CD8αβ cells. We also plan to look at the effect of FTY720, a treatment for MS that sequesters mature lymphocytes into secondary lymphoid tissues and prevents them from migrating to the CNS. An analysis of the potential change in CD8αα abundance following this treatment will allow us to better understand the source of this cell population.
References
Crayton, Heidi J, Howard S. Rossman, Managing the symptoms of multiple sclerosis: A multimodal approach, Clinical Therapeutics, Volume 28, Issue 4, 2006, Pages 445-460, ISSN 0149-2918, https://doi.org/10.1016/j.clinthera.2006.04.005.
Hauser, S. L., Bhan, A. K., Gilles, F., Kemp, M., Kerr, C., & Weiner, H. L. (1986). Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Annals of Neurology, 19(6), 578–587. https://doi.org/10.1002/ana.410190610
Konkel, J. E., Maruyama, T., Carpenter, A. C., Xiong, Y., Zamarron, B. F., Hall, B. E., Kulkarni, A. B., Zhang, P., Bosselut, R., & Chen, W. J. (2011). Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nature Immunology, 12(4), 312–319. https://doi.org/10.1038/ni.1997
Salehi, Z., Doosti, R., Beheshti, M., Janzamin, E., Sahraian, M. A., & Izad, M. (2016). Differential frequency of CD8+ T cell subsets in multiple sclerosis patients with various clinical patterns. PLOS ONE, 11(7). https://doi.org/10.1371/journal.pone.0159565
Sheng, H., Marrero, I., Maricic, I., Fanchiang, S. S., Zhang, S., Sant’Angelo, D. B., & Kumar, V. (2019). Distinct plzf+cd8αα+ unconventional T cells enriched in liver use a cytotoxic mechanism to limit autoimmunity. The Journal of Immunology, 203(8), 2150–2162. https://doi.org/10.4049/jimmunol.1900832
Walton, Clare et al. “Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition.” Multiple sclerosis (Houndmills, Basingstoke, England) vol. 26,14 (2020): 1816-1821. doi:10.1177/1352458520970841

