College of Engineering
25 Temperature Dependence of Leptospirillum Ferriphilum During Biooxidation of Pyrite
Kitsel Lusted; Prasenjit Podder; Kara Sorenson; Prashant Sarswat (Metallurgical Engineering); and Michael Free (Metallurgical Engineering)
Faculty Mentor: Michael Free (Materials Science and Engineering, University of Utah)
ABSTRACT
Rare earth elements have properties that make them useful in advanced electronics, magnets, and batteries. However, they are difficult to isolate from their constituent elements, which results in an environmentally costly operation to refine them. An environmentally friendly alternative extraction method involves biooxidation which uses bacteria to generate acid and ferric ions from pyrite to free the REEs from chemically bonded constituents. Because bacteria are living creatures, one of the most important factors to consider when running a bioreactor is the operating temperature. This study involved testing and analysis of the temperature dependence of biooxidation using pyrite concentrated from coal waste. The temperature was varied from 25°C to 40°C. Acid production in the bioreactor was monitored with pH measurements and bacterial oxidation was measured using the oxidation-reduction potential (ORP) of the system. Bacterial vitality was monitored by periodic ferrous oxidation tests which quantitatively assessed the biooxidation rate. The ferrous biooxidation rate (BOR) was evaluated using the Michaelis- Menten or Monod kinetic equations. Elemental and volumetric mass balances were done after each parameter. Pyrite recoveries were analyzed using energy-dispersive x-ray spectroscopy (EDS), scanning electron microscope (SEM) analysis, and x-ray diffraction (XRD). Additionally, bacteria species analysis showed that Leptospirillum ferriphilum was the dominant bacteria species, showing divergence from the original Acidithiobacillus ferrooxidaans culture. Analysis showed that 35°C had the lowest pH, highest ORP, and highest BOR, while 40°C caused bacterial death.
INTRODUCTION
Rare earth elements (REEs), such as yttrium and lanthanides are widely used in technology and engineering as vital components of cell phones, electronics, and magnets [1]. Contrary to their name, REEs are relatively abundant on the earth’s crust. Previous studies have found that particularly concentrated samples of REEs in coal-mining deposits, which this study focuses on [2]. However, REEs are difficult to refine and extract because they naturally occur with constituent elements [3]. It is, therefore, vital that alternative extraction techniques be explored to sustain the growing demand for REEs while compensating for the decrease in their natural abundance [3].
Solvent extraction is the primary industrial extraction method used to separate REEs from solutions obtained from leaching appropriate ores. Activated carbon and polymeric resin have been explored as a possible alternative to solvent extraction but has not been implemented due to low yield [1]. Solvent extraction in particular uses hazardous chemicals that pose an environmental threat. Traditional solvents, like tributyl phosphate, di-(2-ethylhexyl)phosphoric acid, and Cyanex 272, are effective but toxic and difficult to dispose. Attempts have been made to replace these solvents with benzene, heptane, kerosene, and in some cases, ionic liquids. But many of these replacements show poorer results than traditional solvents and have not been accepted for commercial use [1].
The extraction of REEs using biooxidation has been the subject of vigorous research in the last decades. Biooxidation is the process of using bacteria to free the desired elements from chemically bonded constituents. Since REEs rarely occur in elemental form, biooxidation is easily applicable to the extraction of these metals. This method is particularly appealing because it provides a low-cost, relatively efficient, and non-polluting extraction option [1] [4] [5].
Biooxidation using coal-mining deposits would provide an environmentally friendly extraction method, while also increasing U.S. domestic REE production. A variety of microbes are used in biooxidation however, regardless of the species, these bacteria oxidize the ferrous ions (Fe2⁺) released from pyrite to ferric ions (Fe3⁺). The resulting ferric ions oxidize pyrite and produce sulfuric acid as a byproduct. The bacteria that catalyze the pyrite leaching used in this experiment use a combination of biotic and abiotic reactions:
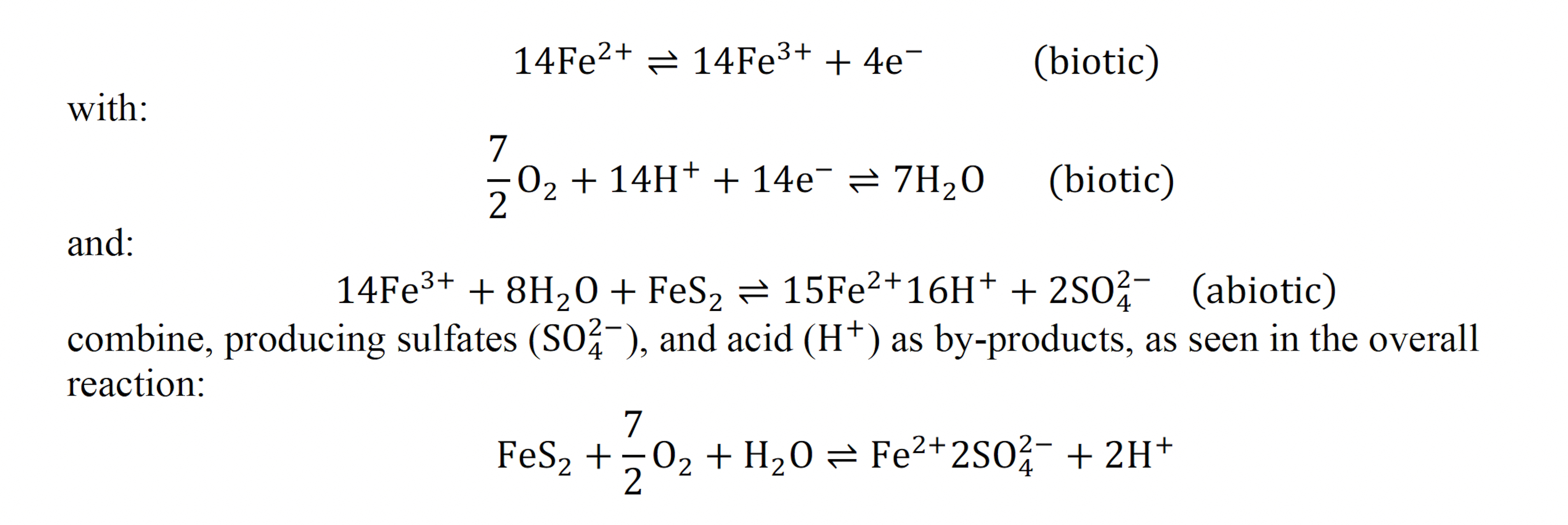
The biooxidation rate is a crucial parameter that measures the bacteria vitality in the bioreactor. It can be measured using the Nernst equation (1), (2), and Michaelis-Menten kinetics (3):
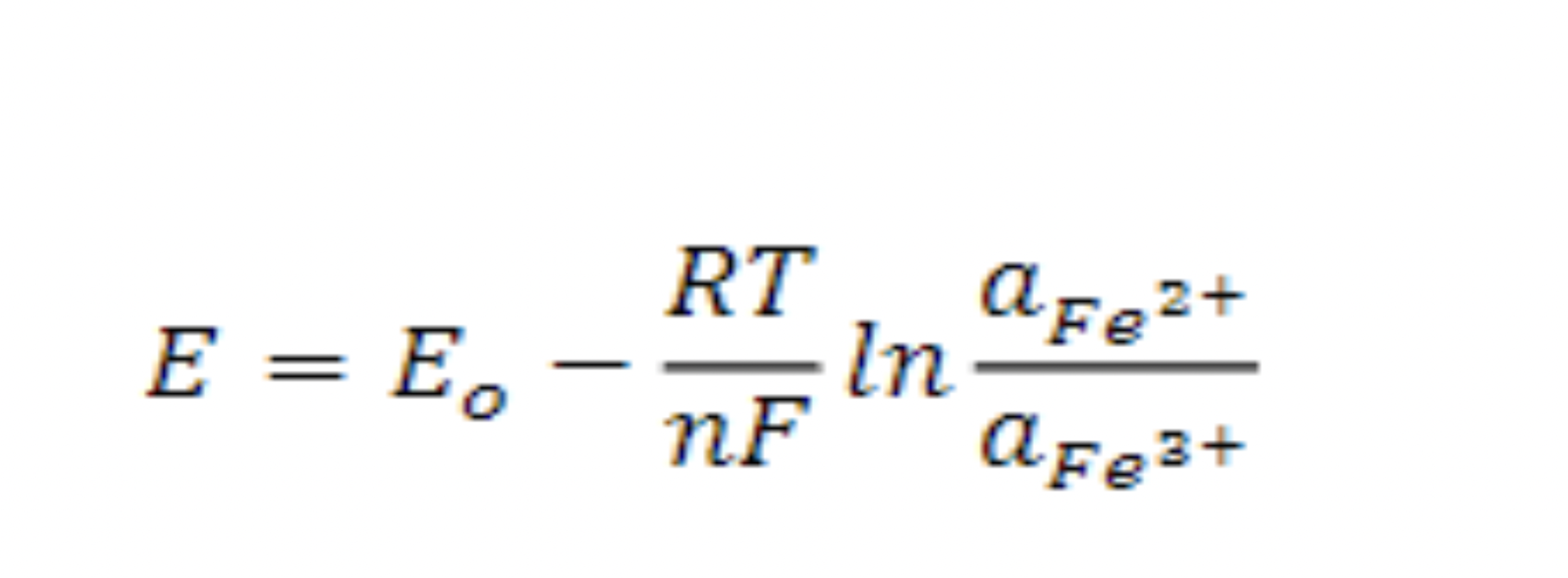
(1)
The electrochemical potential is ‘E’, the standard potential is ‘Eo’, the gas constant is ‘R’, the absolute temperature is ‘T’, the number of electrons is ‘n’, the activity is ‘a’, and the Faraday constant is ‘F’. This equation can be rearranged as:
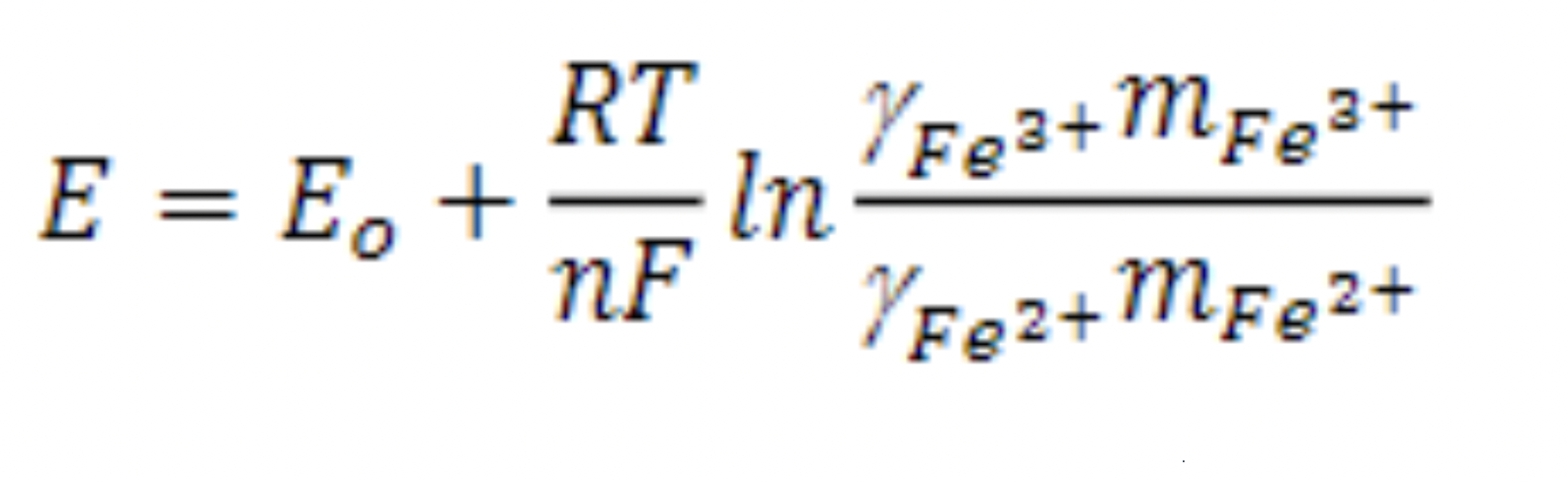
(2)
The initial concentration of ferrous ions can be determined using Michaelis-Menten kinetics. Michaelis-Menten kinetics calculate the rate of biooxidation using the initial (3) and final (4) ferrous ion concentrations.
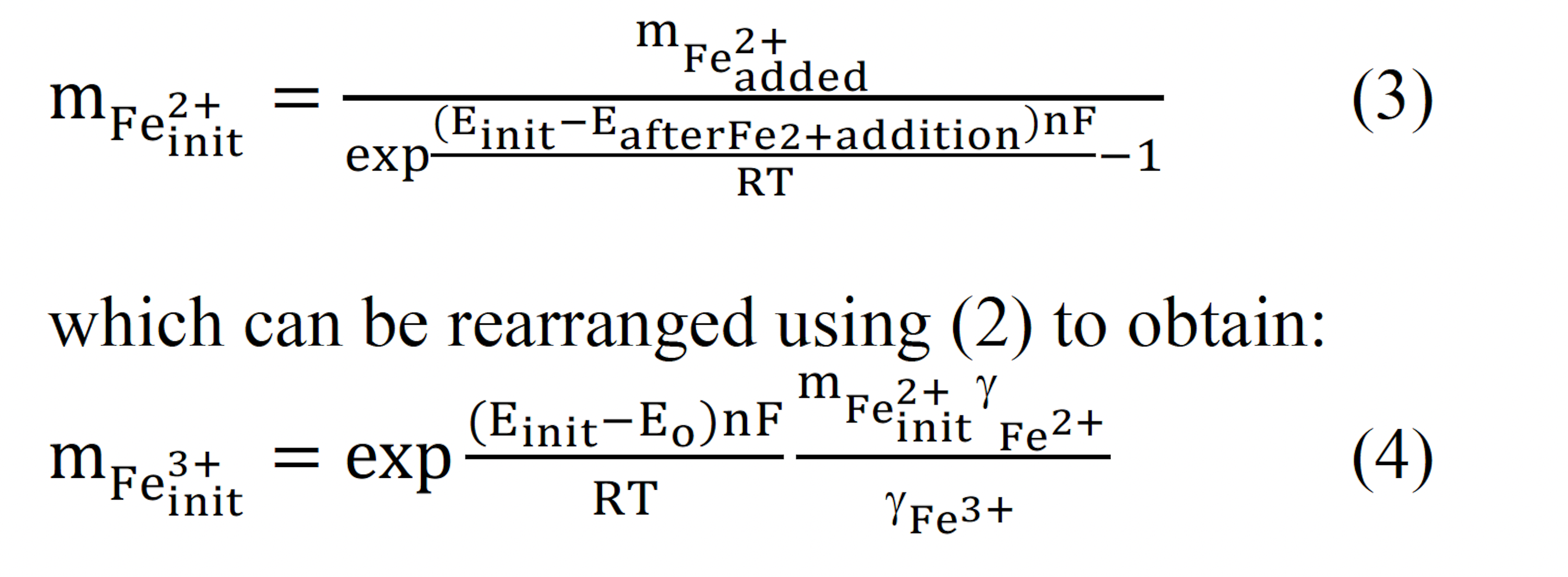
If Eo is assumed to be 0.77 V, the oxidation rate can be expressed as:
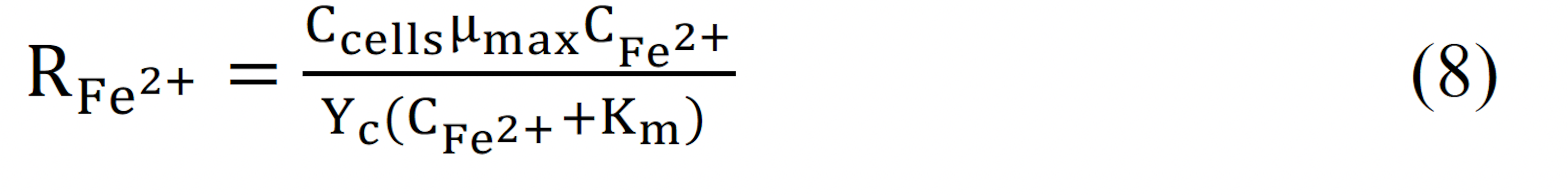
where Yc is the cell yield coefficient, Km is the Michaelis constant, Ccells is the cell concentration and μmax is the maximum specific growth rate. The Michaelis constant was calculated using the Lineweaver-Burke plot.
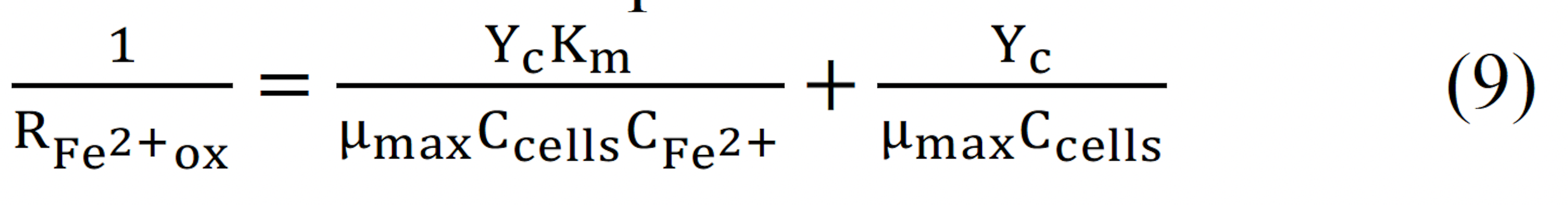
In this study, the rate of biooxidation was tracked by daily measurements of the oxidationreduction potential (ORP), which was reported as the ORP or the electrochemical potential of a redox electrode. Bacterial vitality was monitored by periodic ferrous oxidation testing to quantify the bacterial population by adding a predetermined quantity of ferrous sulfate and subsequent monitoring ferrous ion oxidation. From this data, the level of the bioreactor, the amount of ferrous sulfate added, the temperature of the bioreactor, and ionic strength, the average ferrous oxidation rate can be calculated using a modified Nernst equation (2).
Biooxidizing bacteria operate in extremely low pH (1-2), moderately high temperature, and oxygenated environments [6] [7]. Previous studies have attempted to optimize pH, and oxygen concentration operating parameters for Acidithiobacillus ferrooxidans as well as related microbes such as Thiobacillus ferrooxidans and Leptospirillum ferriphilum [8] [9] [10]. While past studies have presented optimal temperature ranges for biooxidation, there are few conclusive studies that identify the exact optimal temperature for REE extraction [10] [11] [12]. This study investigates the effect of temperature on biooxidation rate, pH, ORP using a biooxidizing bacteria. This study also presents a bacterial species assay of the composition of bacteria within the bioreactor. The results of this study are analyzed using scanning electron microscope (SEM), energy-dispersive spectroscopy (EDS), x-ray diffraction (XRD), and inductively coupled plasma mass spectrometry (ICP-MS) analysis.
METHODS
Set-up A 3L Chemglass bioreactor is attached to a feeding flask and a collection flask. The feeding flask is filled with the feeding solution which varies with each parameter but consistently contains DI water, a slurry of 9K nutrients, and refined pyrite (Figure 1). An overhead stirrer, which is controlled by an automatic timer, is inserted into the feeding flask, and turns on 5 minutes before the scheduled feeding time to ensure that a homogenous solution of liquid and pyrite is pumped into the bioreactor. The feeding slurry is pumped into the bioreactor while solution from the bioreactor is collected and pumped into the collection or effluent flask.
Figure 1: The bioreactor (left) is fed via a feeding flask (right) which contains pyrite, a 9K medium and DI water.


Since the bacteria require oxygen, pressurized air is pumped into the bioreactor through an automatic airflow meter. The bioreactor is kept at a constant temperature by a water bath, where water is heated in the bath and pumped into a closed port of the bioreactor and back into the bath. Thus, the heated water never mixes with the bioleaching solution. The bioreactor is agitated by a ChemGlass overhead stirrer and motor, whose speed is adjustable. Because the instruments that are attached to the lid of the bioreactor raise the level slightly, the volume of the bioreactor used to calculate the average ferrous oxidation rate must be adjusted. In this experiment, if the volume was 2600mL in the bioreactor it was recorded as 2400 mL to account for this difference.
As seen in Table 1, the temperature is varied as residence time, solid weight percentage, and airflow were held constant.
A steady state was established in the bioreactor before data was collected. Once the reactor was operated for approximately two residence times and steady readings for 3 consecutive days were recorded, the data was recorded and treated as representative of the bioreactor’s behavior. The data were averaged over the span of the mass balance.
Acid Generation:
The acid generation of the bioreactor is of particular importance in controlling the homogeneity of the solution. It has been shown in previous studies that predominately jarosite precipitates will form at pH~2.3. These precipitates can form in the bioreactor which inhibits the mechanical ability of the bioreactor and interferes with the bacteria’s ability to oxidize the pyrite. A low pH shows that the bacteria are generating their own acid. It was, therefore, essential to keep the pH below 2.3. If the pH rose above 2.3, it was corrected by adding a small amount of dilute sulfuric acid. The acid production of the bioreactor was monitored through daily pH readings.
ORP and Biooxidation Rate:
The ORP and biooxidation rate (BOR) of the reactor give essential information about the vitality and activity of the bacteria. The ORP and BOR are excellent indicators of mechanical problems inside the bioreactors as they are very sensitive to changes in RPM, airflow, and temperature. For this experiment, only the temperature was varied.
In the context of this experiment, ORP is analogous to the appetite of the bacteria and BOR to the speed at which the bacteria eat. The BOR was calculated by introducing 1g of FeSO4∙7H2O into the bioreactor. The ORP probe was then submerged into the bioreactor. The ORP reading dropped sharply with the introduction of the ferrous sulfate. Once the ORP began to increase, the time for the ferrous oxidation test started. ORP readings were taken every 30 seconds until it reaches within 4-6mV of its starting value. As stated above, the BOR was calculated using the modified Nernst equation (2).
Mass Balance:
Two types of mass balance were performed during this experiment: overall and element-based mass balance. The overall mass balance was calculated after the completion of each parameter by measuring the weight of the effluent solution and comparing it with the mass of the added solution and remaining solution. An average evaporation rate of 7.63% per day was used to calculate the percent yield and determine the efficiency of feed and collection.
For element-based mass balance, effluent and solid samples were analyzed using ICP-MS. ICPMS was done separately on the solid and liquid samples from each steady-state parameter. For a complete picture of the mass balance, the mass balance data were combined in the results. Effluent and solid samples from each steady-state parameter were also analyzed using EDS, SEM and XRD analysis. Before samples went into the XRD machine, they were crushed with a mortar and pestle.
RESULTS
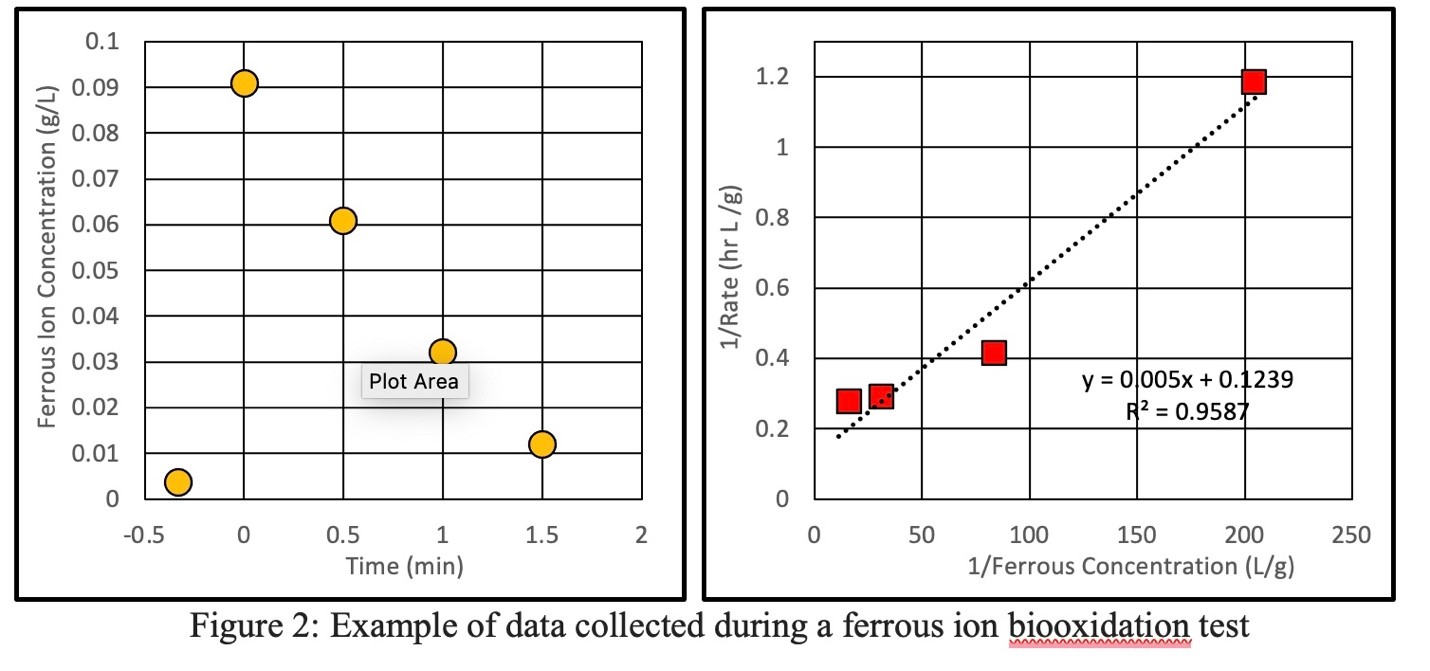
Figure 2 shows the raw data from a sample biooxidation test taken at 35°C. The addition of ferrous sulfate to the bioreactor causes a sharp increase in the presence of ferrous ions in the bioreactor solution as seen in Figure 2a. The biooxidation test starts at time = 0 when the concentration has reached its maximum. As the test goes on, the ferrous ions are consumed by the bacteria and consequently, their concentration decreases to 0. This data is analyzed with Michaelis-Menten kinetics to produce Figure 2b.
Figure 2: Example of data collected during a ferrous ion biooxidation test
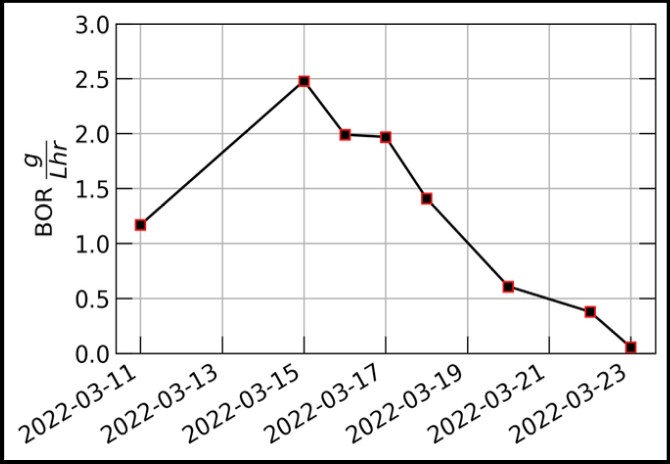
As the temperature increased there were three noticeable trends in the collected data as illustrated in Figure 3. The increase in temperature from 25°C to 35°C caused the average pH to decrease, the average ORP to increase, and the BOR to increase. Figure 4 shows the precipitous decline in BOR recorded during 40°C. There is an initial increase in the BOR from 1.3 7 8∗:; to 2.5 7 8∗:; over the course of four days. This is followed by a drop to 0 7 8∗:; during the next week.
Figure 3: Average pH (A), ORP (mV) (B), and BOR (C) measurements for each steady state parameter
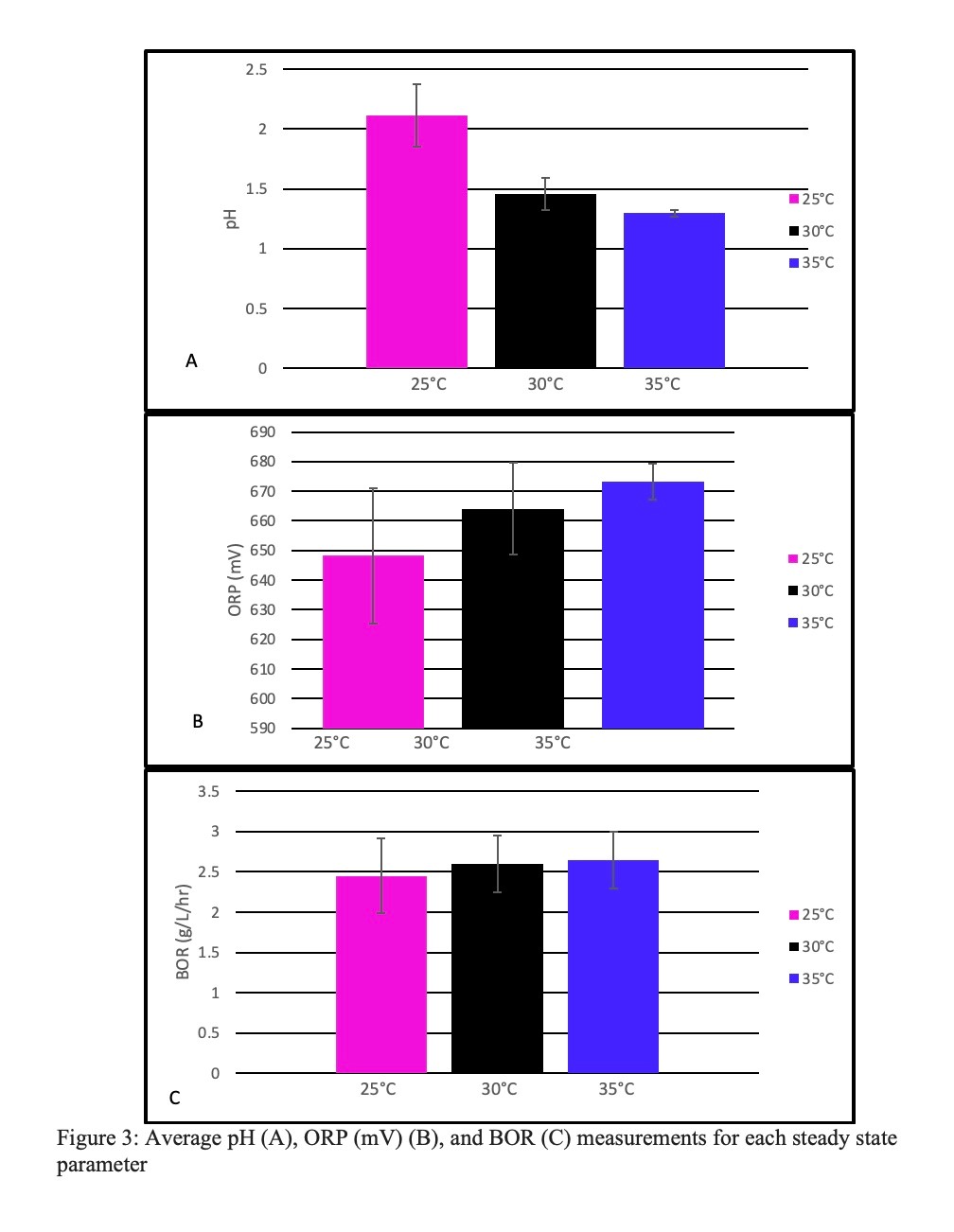
Figure 4: Biooxidation rate measurements for 40°C
The overall mass balance (Figure 5a) shows high efficiency (above 90%) in all parameters. Likewise, element mass balance done with ICP-MS analysis showed a high percent return of all major elements in the solid and liquid sample (Figure 5b). Of the analyzed elements, iron had the lowest return and calcium had the highest overall steady state parameters.
Figure 5: Overall mass balance % efficiency (labeled as success) by weight (A) and element (B) showed high percent success
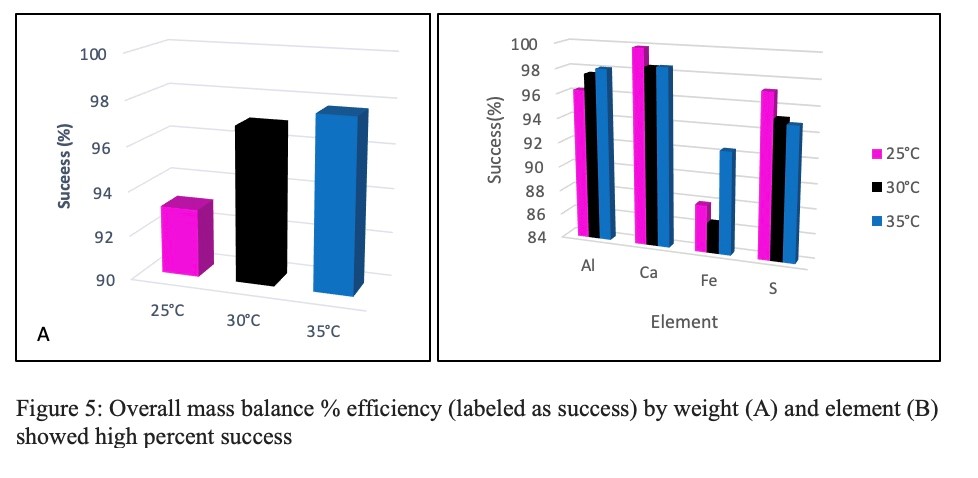
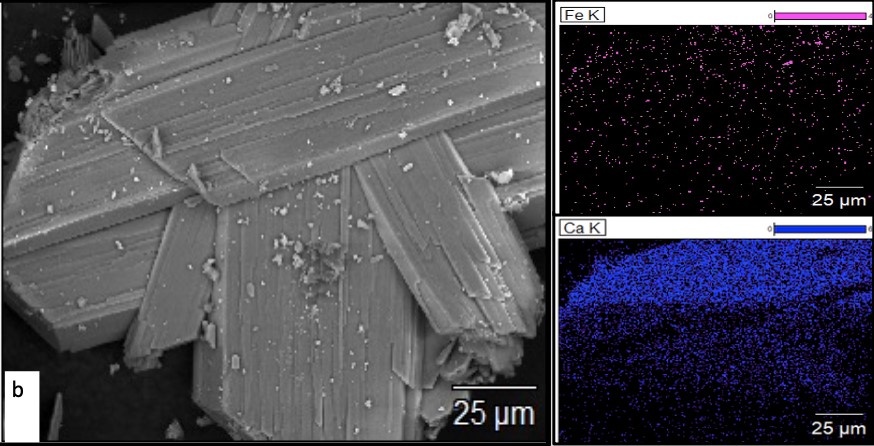
Figure 6 shows SEM/EDX analysis of 25°C and 35°C. These are the lowest and highest temperature parameters that achieved a steady state. 25°C (Figure 5a) is shown at a magnification of 250x. Elemental analysis showed that there is a significant concentration of both iron and calcium on its surface. 35°C (Figure 5b) is at a magnification of 500x. When compared to the elemental concentration of iron in 25°C, 35°C has much less iron on its surface. The concentration of calcium is approximately the same as 25°C. XRD analysis confirmed the high concentrations of calcium, pyrite, and silica in all analyzed samples.
Figure 6: SEM images from 25°C using magnification 250x (a) and 35°C using magnification 500x (b)
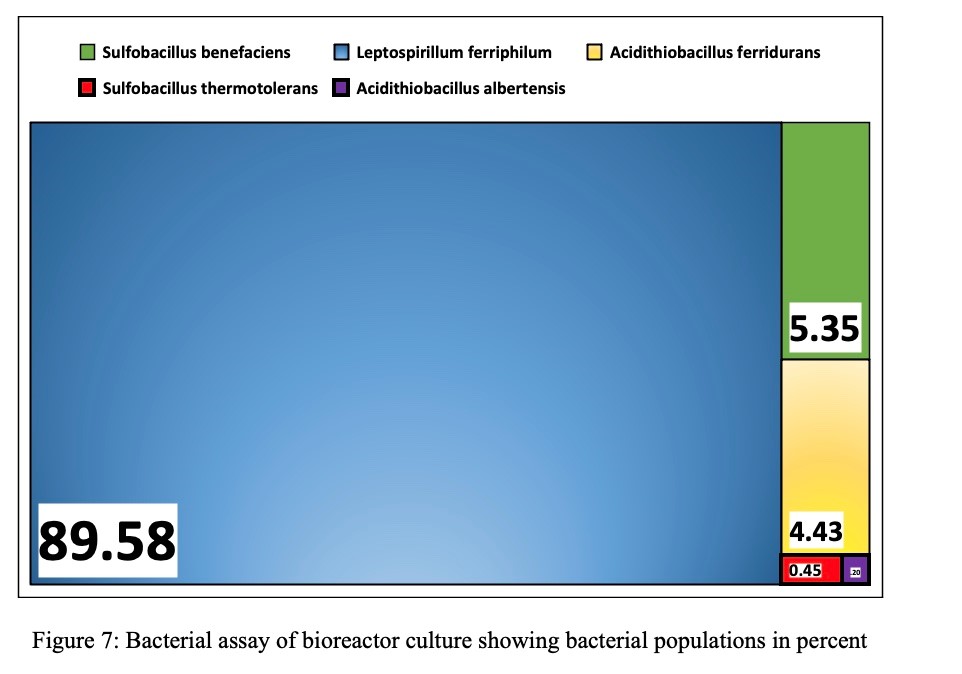
At the conclusion of this study, a bacterial assay was performed on the content of the bioreactor. The results, shown in Figure 7, indicate an overwhelming majority of Leptospirillium ferriphilum (89.58%), with smaller concentrations of Sulfobacillus benefaciens (5.35%) and Acidithiobacillus ferridurans (4.43%).
Figure 7: Bacterial assay of bioreactor culture showing bacterial populations in percent
DISCUSSION
A low pH indicates more acid production in the bioreactor. This acid production aids in the biooxidation of the pyrite resulting in a positive feedback loop of acid production and pyrite oxidation. This is supported by the data shown in Figure 3 where a lower pH leads to more active (higher ORP) and faster-consuming (higher BOR) bacteria. By these standards, the parameter at 35°C showed the best overall performance. There is a larger difference between the gathered [25°C – 30°C] data than between [30°C – 35°C]. This indicates that the minimum running temperature for the bioreactor is 30°C, while the optimal running temperature is 35°C. When the temperature was increased to 40°C, the biooxidation rate dropped dramatically (Figure 4). The initial increase may be attributed to machinery difficulties that were resolved prior to the start of the 40°C parameter. These lingering effects may have contributed to this initial rise, as the bacteria experienced an increase in productivity after several days of no motor agitation. The initial increase was followed by a precipitous drop in BOR over the next week. A zero BOR indicates bacterial death. The bioreactor should not run above 35°C as it leads to inefficient pyrite consumption and eventual death of the bacteria.
The mass balances in Figure 5 show a high yield in both elementally and volumetrically. These results show that the experimental set-up used for all the parameters performed adequately for all recorded parameters.
Figure 6 shows SEM pictures of pyrite crystals from the low and high-temperature parameters (TP 25°C and TP 35°C, respectively). A full EDX analysis was performed, but the most important element in this study was iron since the bacteria consume iron to survive. Conversely, the bacteria do not interact with calcium making it a good comparison element. Visually, the pyrite crystals from 35°C look smoother than those from 25°C. This observation was confirmed with EDX analysis which shows that 35°C has less iron on its surface than TP 25°C. The bacteria were more adept at oxidizing the pyrite at high temperatures as shown in the average BOR measurements in Figure 3. This resulted in the removal of iron from the surface.
At the inception of this study, a culture containing pure, Acidithiobacillus ferrooxidans, were housed in the bioreactor. However, Figure 7 shows that over time the bacterial species evolved to several species with the dominant one being Leptospirillium ferriphilum. During the two-year course of the bioreactor’s performance before this study was conducted, there was no noticeable difference in its performance when held at steady state. Future studies should evaluate the difference and efficiencies between several biooxidizing bacteria to determine whether this evolution is triggered by any one component of the bioreactor’s operating.
CONCLUSIONS
Alternative methods for REE extraction need to be explored due to the growing demand and environmental repercussions of current methods. Temperature is an important factor when extracting REE elements using biooxidation. Using a 3L bioreactor with 6% pyrite and a bacterial catalyst, the temperature was varied between 25°C and 40°C in increments of 5°C. An analysis of the pH, ORP, and BOR data showed that 35°C is the optimal running temperature and results in the highest production of iron and acid from pyrite. 40°C resulted in bacterial death and 25°C produced suboptimal bioreactor performance. This conclusion was supported by SEM and XRD analysis of the pyrite crystals. Future work will include the analysis of rotation speed, bacterial culture, and solid percent on the performance of the bacteria.
REFERENCES
[1] N. Hidayah and S. Abidin, “The evolution of mineral processing in extraction of rare earth elements using solid-liquid extraction over liquid-liquid extraction: A review”, Minerals Engineering, 112 103-113 (2017).
[2] S. Massari and M. Ruberti, “Ra [13] [13]re earth elements as critical raw materials: Focus on international markets and future strategies”, Resources Policy, 38 [1] 36-43 (2013).
[3] L. Ferreira, T. Müller, M. Cargnin, C. de Oliveira and M. Peterson, “Valorization of waste from coal mining pyrite beneficiation”, Journal of Environmental Chemical Engineering, 9 [4] 105759 (2021).
[4] Cockell, C.S., Santomartino, R., Finster, K. et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat Commun 11, 5523 (2020).
[5] A. Mazuelos, N. Iglesias and F. Carranza, “Inhibition of bioleaching processes by organics from solvent extraction”, Process Biochemistry, 35 [5] 425-431 (1999).
[6] R. Quatrini and D. Johnson, “Acidithiobacillus ferrooxidans”, Trends in Microbiology, 27 [3] 282-283 (2019).
[7] J. Valdés, I. Pedroso, R. Quatrini, R. Dodson, H. Tettelin, R. Blake, J. Eisen and D. Holmes, “Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications”, BMC Genomics, 9 [1] (2008).
[8] S. MOUSAVI, S. YAGHMAEI, F. SALIMI and A. JAFARI, “Influence of process variables on biooxidation of ferrous sulfate by an indigenous Acidithiobacillus ferrooxidans. Part I: Flask experiments”, Fuel, 85 [17-18] 2555-2560 (2006).
[9] S. Mousavi, S. Yaghmaei and A. Jafari, “Influence of process variables on biooxidation of ferrous sulfate by an indigenous Acidithiobacillus ferrooxidans. Part II: Bioreactor experiments”, Fuel, 86 [7-8] 993-999 (2007).
[10] S. Mohapatra, S. Bohidar, N. Pradhan, R. Kar and L. Sukla, “Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferrooxidans and Aspergillus strains”, Hydrometallurgy, 85 [1] 1-8 (2007).
[11] S. Malhotra, A. Tankhiwale, A. Rajvaidya and R. Pandey, “Optimal conditions for biooxidation of ferrous ions to ferric ions using Thiobacillus ferrooxidans”, Bioresource Technology, 85 [3] 225-234 (2002).
[12] C. Montes-Rosua, N. Iglesias-Gonzalez, A. Mazuelos, R. Romero and F. Carranza, “The effect of temperature on the bio-oxidation of mining effluents containing tetrathionate”, Hydrometallurgy, 178 37-42 (2018).k

