Science
93 Spectroscopic Studies of Lanthanide Nitrides
Yexalen Barrera-Casas; Michael Morse (Chemistry); and Dakota Merriles
Faculty Mentor: Michael Morse (Chemistry, University of Utah)
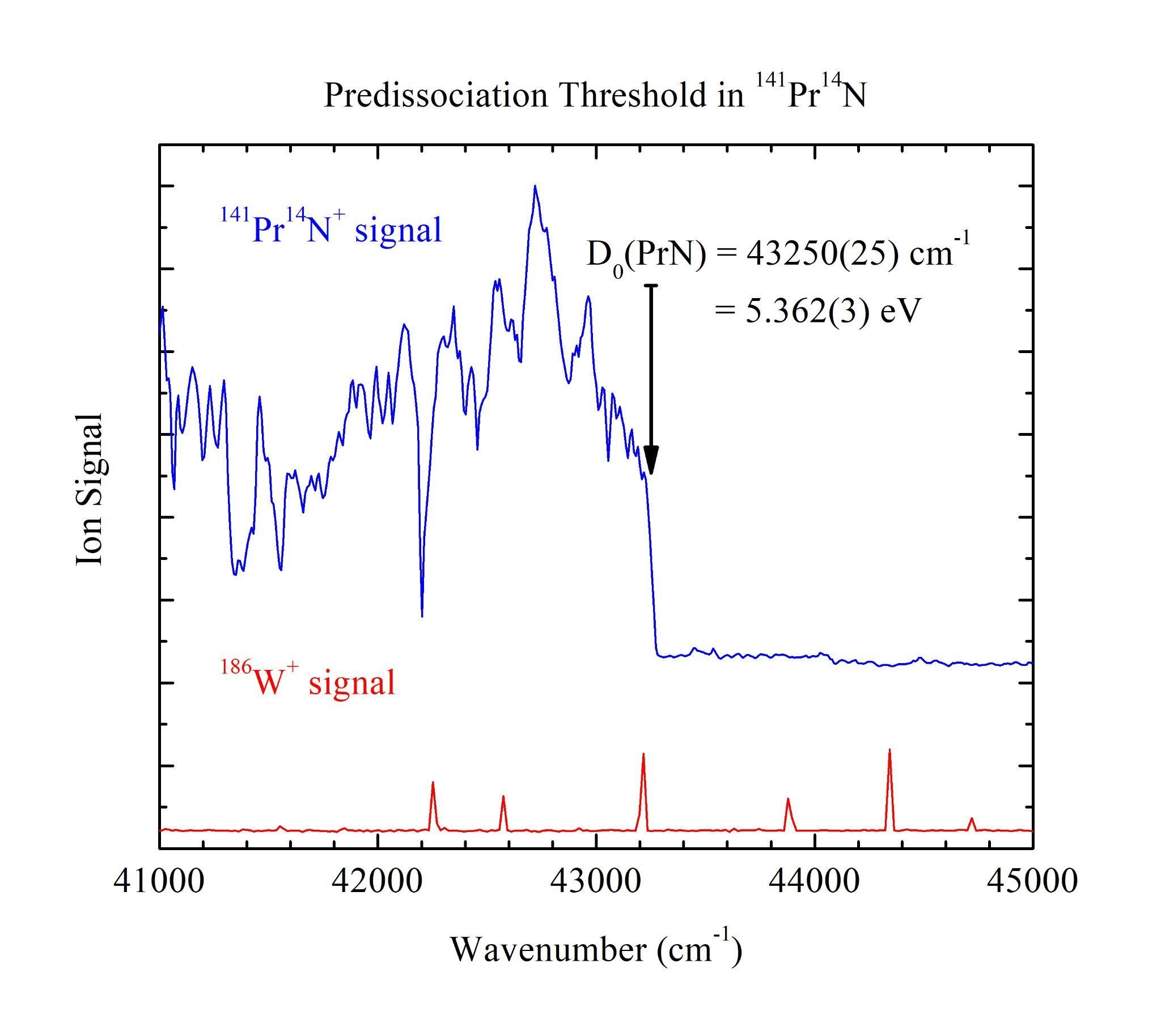
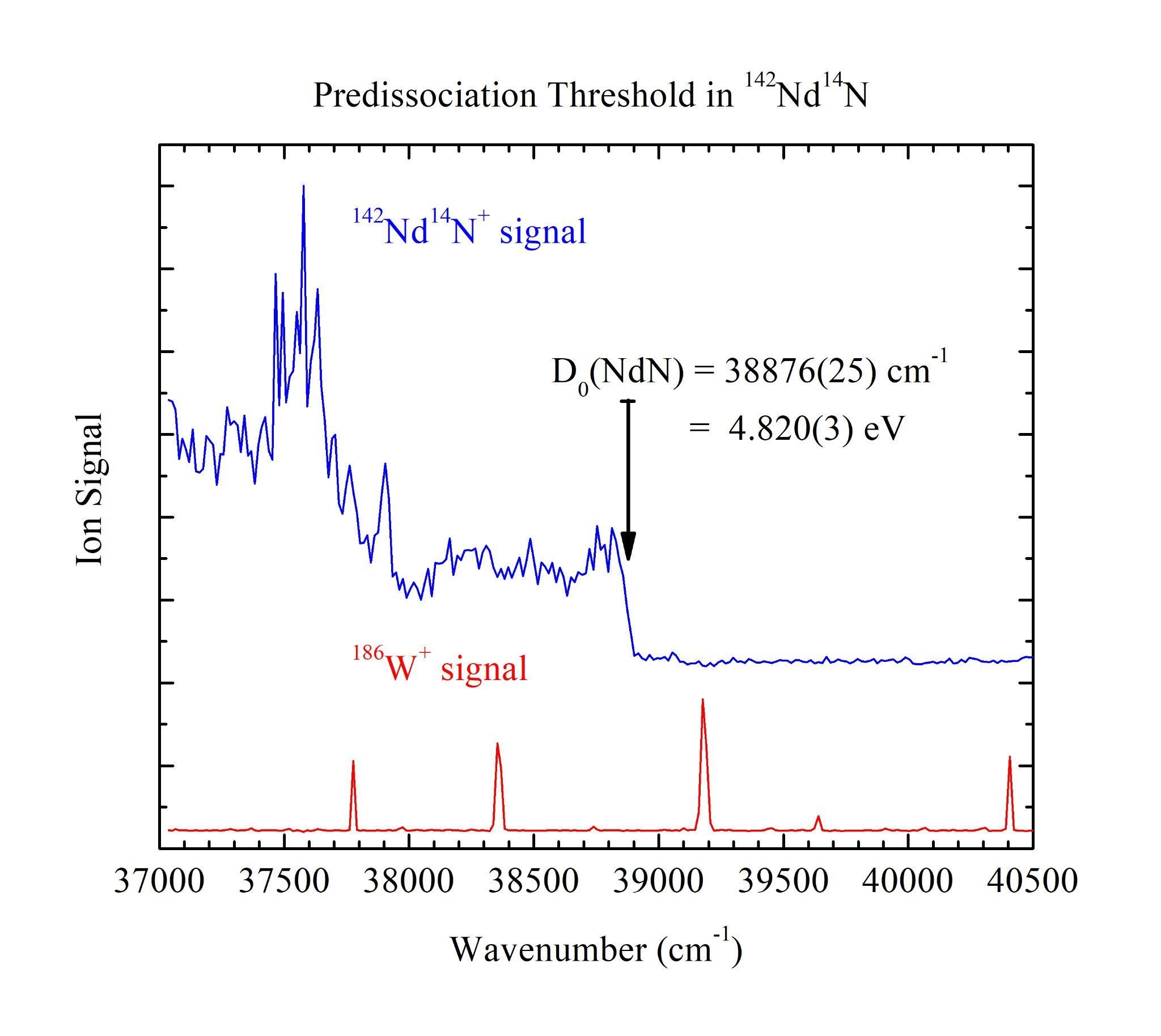
With the employment of Resonant Two-Photon Ionization (R2PI) spectroscopy and jet-cooled molecular beams, the sharp predissociation thresholds of lanthanide metal nitride diatomics (LnN, Ln = Pr, Nd, Gd, and Tb) were measured. These species do not have previously reported bond dissociation energy (BDE) measurements in the literature which prevents the classification of important periodic trends and overall fundamental characteristics. One of the most informative fundamental characteristics of a molecule is its BDE, which is the amount of energy required to bring a molecule from its ground vibronic state to its ground separated atom limit. The first portion of the R2PI spectra illustrates a congested manifold of molecular vibronic states at energies below the ground separated atom limit. The second portion of the spectra is resolved when the molecule of interest is excited to energies at or above the ground separated atom limit. Here, the molecule rapidly dissociates and the measured ion signal drops to a baseline indicating zero molecular signal. The BDE can be accurately and precisely assigned at the transition from molecular signal to baseline. The BDEs of the LnN studied in this work are D0(PrN) = 5.362(3) eV, D0(NdN) = 4.820(3) eV, D0(GdN) = 4.273(3) eV, and D0(TbN) = 4.261(3) eV. These results help illuminate the fundamental principles of the chemical bonds between these lanthanide metals and nitrogen.
About the authors
name: Yexalen Barrera-Casas
institution: University of Utah
name: Michael Morse
institution: University of Utah
name: Dakota Merriles
institution: University of Utah

