School of Medicine
72 My Lung Health: Shared Decision Making For Lung Cancer Screening
Grace Richards
Faculty Mentor: Victoria Lynn Tiase (Biomedical Informatics, School of Medicine, University of Utah)
ABSTRACT
Lung cancer is the deadliest cancer in the United States. In 2019, around 140,000 deaths occurred in the Untied States due to lung cancer. Low-dose CT screening to detect lung cancer is an effective tool for preventing these deaths, but the screening has some harms associated with it in addition to the benefits. It has been established that patients have better outcomes when information about lung cancer and low-dose CT lung cancer screening is readily available to them. This allows them to participate in effective shared decision-making (SDM) regarding the decision to be screened for lung cancer. To facilitate effective SDM, SDM tools must be accessible to patients as well as clinicians. Despite this knowledge, a comprehensive patient-facing tool for lung cancer screening shared decision making is not currently available in many settings. We intend to inform the design of a doctor-prescribed, patient-facing mobile health tool to provide patients with information about their risk for lung cancer and the lung cancer screening process. We explore the user needs, perceived usefulness, and perceived ease-of-use of a mobile-based shared decision-making tool for patients. Our methodology included focus groups and surveys to solicit patient’s needs and assess their perception of usefulness and ease-of-use. Overall, the participants’ feedback supports the further development of a tool. We found many valuable recommendations to improve the usefulness and ease-of use of a patient-facing SDM tool.
The recommendations will be used to inform the next steps in the development process. Including patient perspectives (user needs) in the design of a web-based tool has the potential to empower patients in the participation of SDM and lung cancer screening, leading to better health outcomes and decreasing mortality from lung cancer in the U.S.
INTRODUCTION
Lung cancer screening is one of the most effective cancer screenings in the United States due to its potential to save 10,000 lives per year; however, it is also one of the most underutilized, with only around 5% of eligible patients being screened [1]-[2]. This is especially true in minority and underserved populations [3]. One way to increase lung cancer screening rates among eligible patients is to implement informatics tools that guide shared decision-making (SDM) between patients and providers [4]-[5].
Shared decision making (SDM) is defined as: “…a process in which both the patient and healthcare professional work together to decide the best care plan for the patient,” [6]. This process makes a special effort to consider the patient’s values, goals, and preferences in contrast to traditional clinical decision-making. The use of SDM in clinical practice occurs when both the patient and the provider are informed about the risks and benefits of the treatment for the patient personally and all the potential options for care regarding the medical treatment in question [7]-[8]. SDM is considered the standard when it comes to clinical practice regarding decision making about lung cancer screening and is encouraged by many national and international organizations including the US Preventative Services Task Force which recommends that LCS should not occur without a SDM process [5], [9]-[12]. In fact, the Centers for Medicare and Medicaid Services (CMS) requires that SDM used with patient decision aids for eligible patients be covered since 2015 [9]-[10]. The reason why SDM is particularly useful in lung cancer screening decisions is that the variability in the risks and benefits from one patient to the next depending on age, years of smoking, exposure to carcinogens, and other patient measures [9], [13]-[14]. Thus, a simple “yes/no” approach to lung cancer screening does not sufficiently account for patient preference, and a more nuanced, risk-benefit analysis is required for true shared decision-making. While it has been shown that clinicians, too, prefer a SDM process in patient decision making, especially in lung cancer screening [15], in actual clinic visits they often do not reach this standard of care [9], [16]-[19]. Based on an expert panel from the American College of Chest Physicians (CHEST), low-dose CT screening for lung cancer is beneficial procedure but has a “tenuous balance of benefit and harm,” that is patient sensitive [14], [20]. Therefore, decision aids that include risk prediction calculators are becoming increasingly preferred in lung cancer screening guidelines [12], [21].
One example of an SDM tool is ScreenLC (Center for Health Communications Research, University of Michigan, Ann Arbor, MI), an electronic web-based decision aid utilized by providers at the University of Utah Health. This application guides a provider in understanding a patient’s risk profile and includes an individualized risk calculator (Fig. 1). The tool is meant to be reviewed by the provider with patients during the patient visits. However, the clinician’s knowledge of the risks and benefits of the procedure is not enough. To have an effective SDM process, patients must also be empowered to understand and make decisions together with their providers. Many patients, unfortunately, do not have access to information about their risks and benefits in advance of an appointment with their provider [51]. Anecdotally, providers have requested that patients access the information provided by ScreenLC in the form of a prescribed informatics tool before meeting to discuss lung cancer screening.
Our team is investigating the design and development of a new mobile-based tool that patients would have access to at any time after it is prescribed by their provider, both prior to a visit and after they see their provider. The tool could benefit from elements such as information adapted to a health illiterate population, effective and succinct delivery of personalized information, and most importantly, encourage questions for the SDM process between the patient and a provider [17], [18], [52]. The user requirements, usefulness and ease-of-use of such a tool is still uncertain, necessitating continued research of the topic.
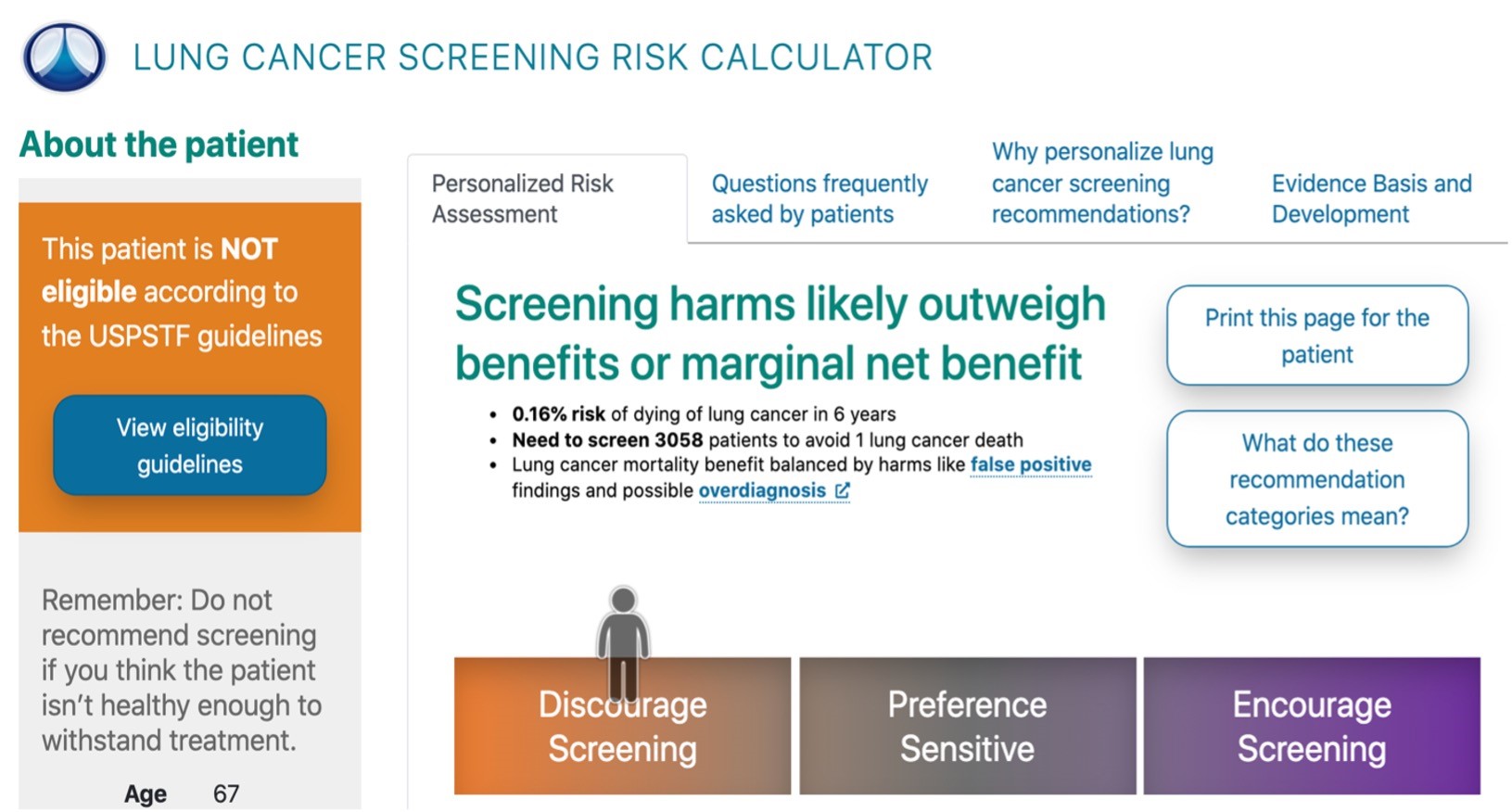
Figure 1.A screening recommendation from the ScreenLC tool for providers with example patient demographics from ScreenLC.com
The goal of the overarching project is to empower patients to make informed decisions about their lung health. The larger study aims to build a web and mobile-based application to aid patients in understanding the risks and benefits of lung cancer screening and update their smoking history within their medical records. The new mobile-based tool will be based on the ScreenLC application. It will be modified from ScreenLC to a patient-facing application accessible at any time to prepare for SCM discussion about lung cancer screening. We want to ensure the application is culturally appropriate for at-risk populations, specifically African Americans and Hispanics.
The research outlined in this thesis describes user feedback related to the usefulness and ease-of-use of the current clinician focused tool as well as the perceived usefulness and ease-of-use of a patient-facing version. We conducted focus groups and surveys to collect qualitative and statistical quantitative data regarding patient’s views of ScreenLC as if it were to be a patient-facing version of this application. The results will be used to provide recommendations for the design of a patient facing SDM tool. In turn, we anticipate that this research will support the design of SDM tools that empower a broad population of patients to make informed decisions about lung cancer screening. Ultimately, the resulting SDM may improve the lung health of our population by increasing the screening rate of eligible patients leading to fewer deaths nationwide from Lung Cancer.
BACKGROUND
Lung cancer screening is an underutilized preventative measure with the potential to save many lives. Despite the US Preventive Services Task Force recommending low-dose CT screening for eligible patients, adoption is low [2], [22]. They recommend screening for anyone who currently smokes or has quit within the past 15 years, has a more than 30-year smoking history, and is between 55-80 years of age [5], [22]. These guidelines were based on the National Lung Screening Trial (NLST). The NLST was a seven-year trial launched in 2002, where 53,454 patients were randomly assigned to low-dose CT screening or chest radiography to determine the effectiveness of CT screening. The low-dose CT had a 20% reduction in mortality compared to the chest radiography group [5], [23]. Based on this study, it is estimated that 10,000 lives could be saved each year if eligible patients in the top 60th percentile for risk of lung cancer were screened. This trial and the recommendations from the U.S. Preventive Services Task Force establish the credibility and guidelines for screening. Unfortunately, less than 5% of eligible people currently undergo screening for lung cancer [1], [2]. Lung cancer is the deadliest cancer, accounting for 25% of cancer deaths [1], [24]. Despite this, the screening rate of eligible patients for lung cancer is much lower than the screening rates for other cancers, with the screening rates in 2019 being 76.4% for breast cancer, 37.0% for prostate cancer, and 68.8% for colorectal cancer [24]-[25]. The reason for this is multifactorial, but one barrier to screening is eligible patients lacking accurate information [21].
Despite the benefits of screening, deciding to be screened for lung cancer is not a simple choice for even eligible patients. Low-dose CT screening has potential risks. There is a high false-positive rate in CT screening, resulting in patients receiving unnecessary and possibly harmful procedures [13], [26]. There can be long-term health effects from radiation exposure [27]-[28]. Additionally, patients need to be screened annually which is a long-term commitment to care, and a financial burden that many patients are not willing or able to commit to [29]. Whether or not a patient should be screened for lung cancer varies greatly depending on the patient. This decision is dependent on a patient’s smoking history, exposure to secondhand smoke and asbestos, and other demographic circumstances. Because of the varied risk, SDM between a provider and a patient is strongly suggested when deciding whether a patient should be screened for lung cancer. Accordingly, CMS requires the use of SDM for at-risk patients to be covered for screening [11]-[12].
A decision tool to facilitate SDM by providing patients with evidence-based, personalized information about options, risks, benefits, and costs of a medical treatment is recommended [8]. The purpose of SDM tools is to ensure that patients are supported, well-informed and have ownership over their healthcare. ScreenLC is an example of an electronic decision aid that provides this kind of information to clinicians to encourage informed discussions with patients regarding screening for lung cancer. It is integrated into the University of Utah’s electronic health record. However, one key aspect of SDM: giving the patient access to this information directly and prior to their visit to access at any time, is still missing [21].
Patients must have access to personalized and evidence-based information about lung cancer screening to be fully engaged in the SDM process [16]. In preliminary discussions with clinicians who currently use ScreenLC, there is a desire to have patients access the same information that the clinicians are privy to in order to ensure that their patients are well informed before and after their visit. The intent is to reduce the stress of difficult decision-making by preparing the patient with information ahead of the visit, like whether to be screened for lung cancer.
There is little known regarding the user needs for such a tool. However, it has been established that comprehensive information to meet the high information needs of the target user population is important for a SDM tool [17], [18], [21]. In general, patients want more information regarding screening decisions. In addition, an interactive tool that uses plain language has been shown to be favorable [17]. A few lung cancer screening tools have shown high levels of general acceptability, but the user requirements are lacking [17], [18], [52].
Underserved and minority populations have low screening rates and higher mortality from lung cancer [3]. Additionally, as more healthcare interventions are moved online, there is a growing concern about the inequities in access to these healthcare tools between majority and minority groups [30]. We aspire to address this increasing divide by making the mobile-based patient facing application easily accessible and adapted to minority populations. As part of this goal, we have ensured these groups are represented in our study and incorporate their feedback into the development of our tool.
METHODS
To build an SDM tool in a clinical setting, first, it was necessary to gather user needs and assess the perceived usefulness (PU) and perceived ease of use (PEU) of the tool. Perceived usefulness measures to what extent potential users of technology perceive that the technology would enhance their experience in a particular domain, in this case the domain is their lung health [31]. Perceived ease of use measures the degree to which a potential user perceives that using a particular tool would be free from effort [31]. Our procedures included a literature review, consulting with domain experts, and soliciting input from target users through a focus group and a survey. Focus group participants were shown the ScreenLC application with a demonstration of the personalized risk calculator. The potential for adaption to a patient-facing tool was described. Participants were asked for verbal feedback through focus groups and quantitative feedback with a post-focus group survey.
A. Literature Review
For our literature review, hundreds of papers related to cultural adaption of informatics tools, SDM and SDM decision tools were reviewed. The process allowed us to survey the current literature, note any gaps in the standard of care, and establish a framework for our focus group and survey. We extracted the author, year, title, abstract, principal/relevant points, and a rating for relevance for each paper. Our first inclusion criteria was that the topic of the paper must be cultural adaption, shared decision making informatics tools, or shared decision making. Our second inclusion criteria was that the paper must be published in the past 10 years. We excluded papers that were not related to informatics tools.
B. Focus Group Questions, Script
Our purpose statement for our focus group and post focus group survey was to better define the general user needs and the PU and PEU of a web-based tool to inform the further design and 10
development of the patient-facing tool.
To plan and finalize details of the focus group the team consulted experts, including community engagement resources at the University of Utah. The inclusion and exclusion criteria, the recruitment process, the number of participants and number of focus groups, and the duration of the focus group were finalized. A REDCap (Vanderbilt University, Nashville, TN) survey (Appendix: Table 1A) was developed for collection of demographic data and screening participants.
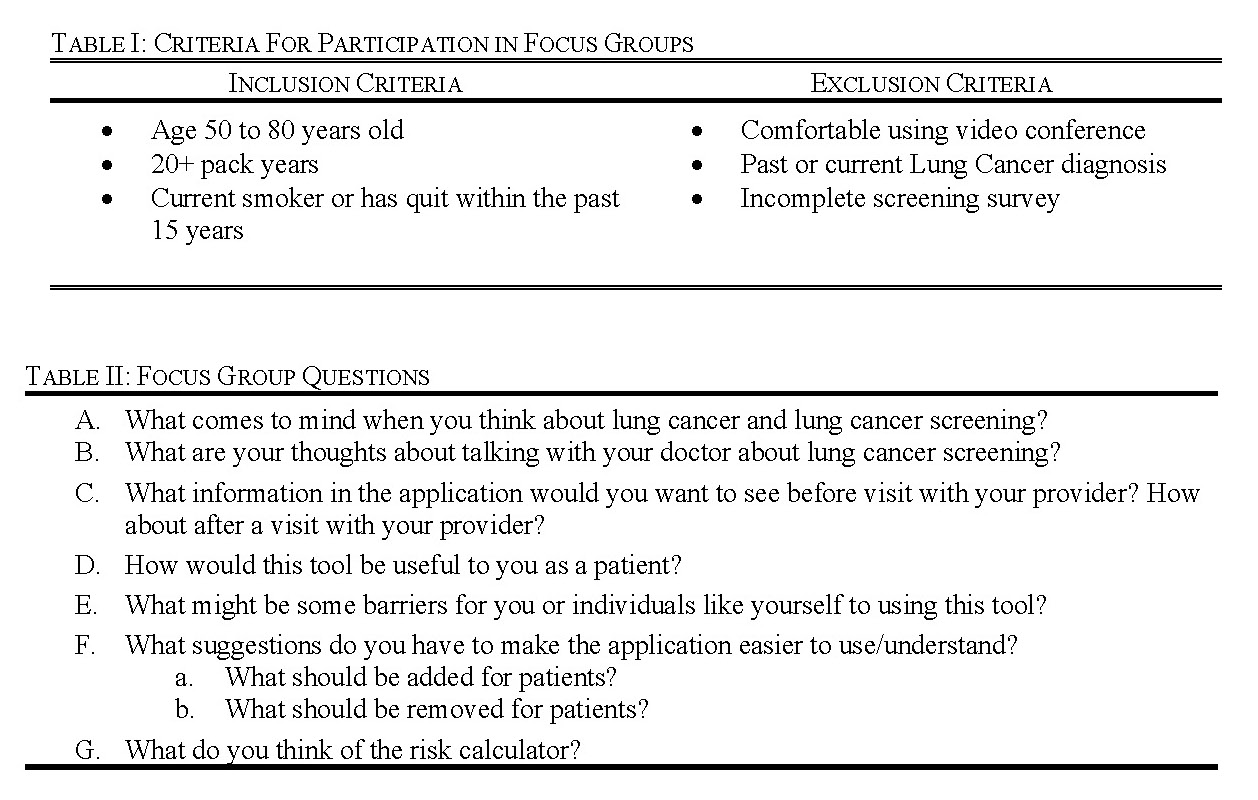
The inclusion criteria were developed based off the USPSTF screening guidelines, which are the guidelines recommended by the American Cancer Society (ACS) [12]. The exclusion criteria were developed based on the constraints of the focus group. Potential participants were excluded if they did not self-identify as ‘very comfortable’ or ‘somewhat comfortable’ with video conferencing (Table I). They were also excluded if they did not complete the screening survey including all of the demographic information (Appendix: Table A1).
Our goal for each group was to include at least two individuals who self-identified as Black and at least two individuals who self-identified as Hispanic.
An original draft of the focus group questions that would serve as a basis for the facilitator’s script during the focus group were created using a framework similar to the ‘Sample Focus Group Moderator’s Guide’ in to Making Health Communication Programs Work [32]. Specifically, the section ‘Steps in Developing and Pretesting Messages and Materials’ (pg. 185) was used to guide our development of the focus group process. The first draft consisted of 28 questions. Through various iterations and expert consensus, we finalized seven questions for the focus group (Table II).
C. Post-Focus Group Survey Development
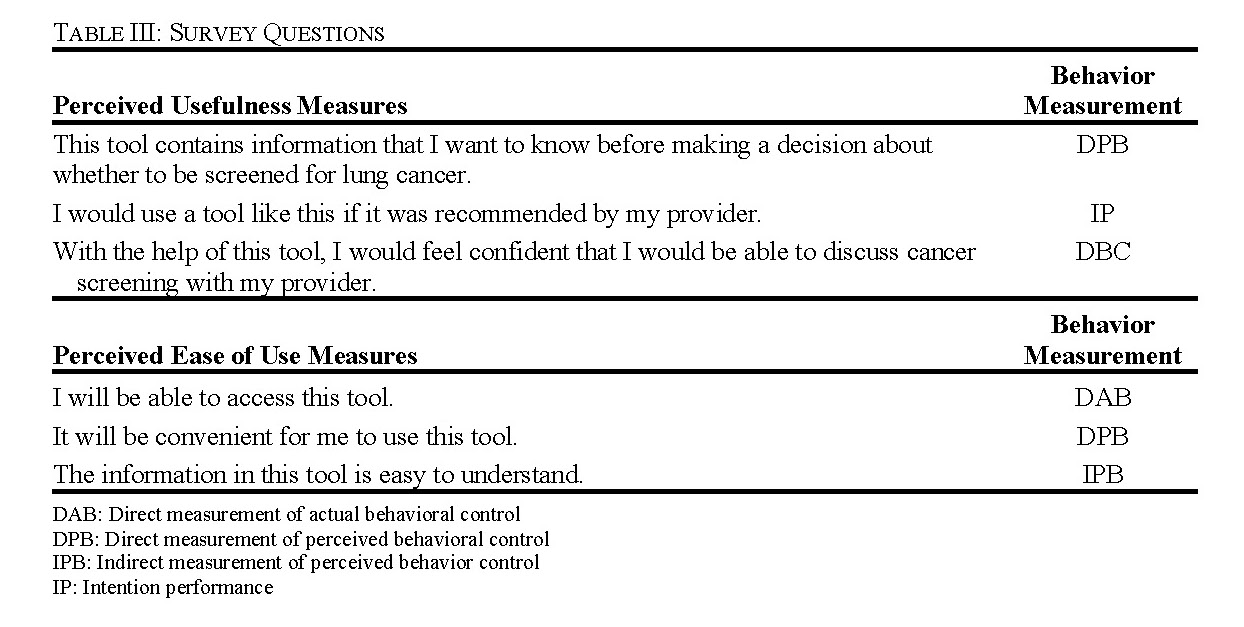
We began by selecting six measures related to the perceived usefulness (PU) and perceived ease of use (PEU) for the post-focus group survey. These measures were informed by the Technology Acceptance Model (TAM) questionnaire as a general framework [31], [34]. The questions were modified further using ‘Constructing Questionnaires Based on The Theory of Planned Behavior’ [33] and close collaboration with Dr. Kimberly Kaphingst (University of Utah), an expert in health communications. Based on the Theory of Planned Behavior, the behavior in question for our study was a participant’s use of the tool. There were several drafts that were reviewed by three members of the team including Dr. Kaphingst before finalization. We included six questions total with three of the questions aimed at measuring perceived usefulness (PU) of the tool and three questions aimed at measuring perceived ease of use (PEU) of the tool (Table III).
For each question, respondents would rate their ‘percent agreement’ on a scale from 0 to 100 with 100 being full agreement, 50 being neutral, and 0 being full disagreement (Fig. 2). 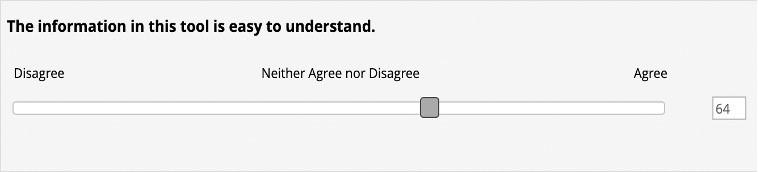
Figure 2. Display that respondents saw while filling out the post-focus group survey with a scale that goes from 0 to 100 with 100 being full agreement.
C. Focus Group Procedures
The methods for focus group recruitment included direct outreach through collaboration with the Huntsman Cancer Institute’s (Salt Lake City, UT) Health Outcomes and Population Equity (HOPE) program for tobacco cessation, and the University of Utah’s Community Collaboration & Engagement Team. We also distributed flyers to Facebook (Menlo Park, Ca) pages, in person at University of Utah clinics, and ResearchMatch (Vanderbilt University, Nashville, TN). We contacted individuals who met our inclusion criteria in the HOPE program database through email to be a part of our focus group. Each person received a QR code linked to the REDCap screening survey (Appendix).
The focus groups met for a recorded video conference session over Zoom (Zoom Video Communications, San Jose, CA) that lasted approximately 2 hours in duration. The focus groups took place in the second half of 2022 and early 2023.
After introductory remarks, consent review, and discussion about questions A-B (Table II) participants were given a short presentation of the tool and provided some background information about lung cancer and low-dose CT screening (Fig. 3). They were also provided with an example demonstration of how a patient would use the risk-calculator (Fig. 3). The facilitators then asked the participants the focus group questions C-G (Table II). At the completion of the focus group, participants were asked to complete post-focus group survey. All participants were compensated for their time.
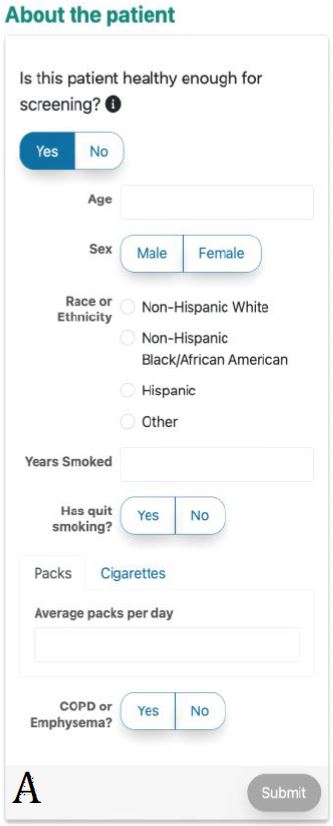
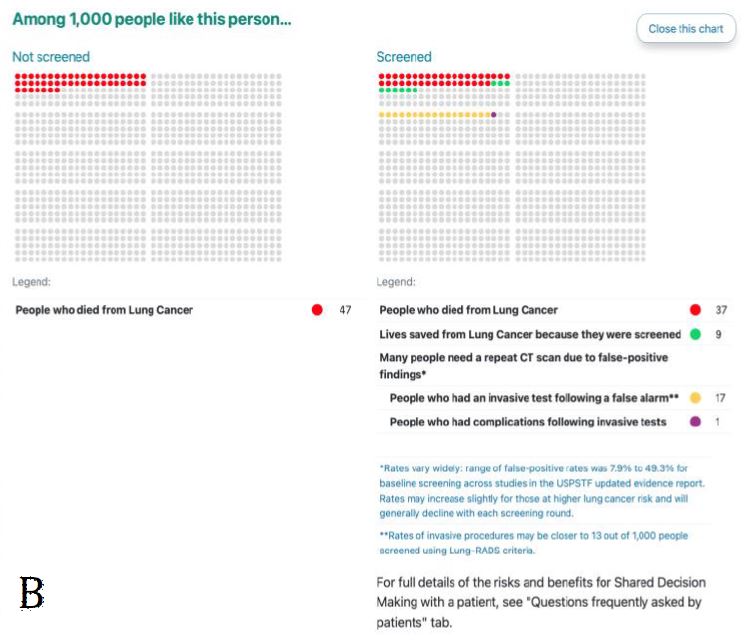
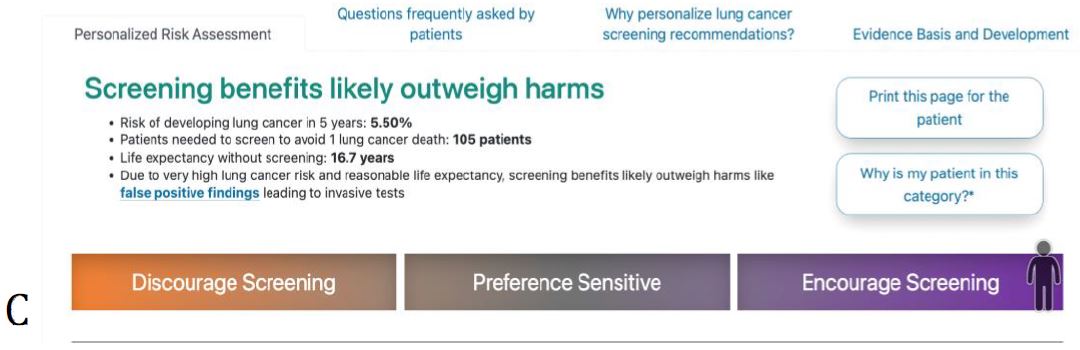
Figure 3. ScreenLC Display. [A] The data entry user interface screen, this is the first page that a user of ScreenLC sees [B] A visual representation of the patient’s risk profile using a dot probability display [C] The personalized screening recommendation for the Patient
Upon completion of all four focus groups, zoom audio recordings were downloaded and securely transmitted to a transcription service. The deidentified transcripts were uploaded into Dedoose (dedosse.com, Inc.). The team conducted a thematic analysis by coding participant responses through consensus and then identified themes and patterns. From the themes and 15
patterns, recommendations were formulated for the design and development of a patient facing SDM tool.
- D. Statistical Analyses
The results from the post-survey were analyzed using descriptive statistics. These results were reported with the mean score and standard deviation for each question. In addition, for each measure the number of respondents that agreed, neither disagreed or agreed, and disagreed with the statement were reported. Participants who responded with a score higher than 60% were considered to be in agreement with the measure, 60-40% was considered neutral, and less than 40% was considered disagreement.
IV. RESULTS
A. Literature Review
We included 19 papers in the literature review [8]–[10], [19], [35]-[48]. Many papers are concerned with the cultural adaption of informatics tools due our original intention to adapt ScreenLC to be more culturally appropriate for Hispanic populations and translate the tool into Spanish [35]-[36], [38]-[44], [46]-[47]. However, through our literature review and anecdotal feedback from clinicians, we determined that there was a greater need to adapt the tool to be patient-facing first. Another major takeaway from the literature review was that SDM does improve patient outcomes but adoption and facilitation of SDM varied widely [8]-[10], [19], [45]. Many papers found that patient empowerment through information and education was key to successful SDM [9], [37], [45].
B. Focus Group Participant Demographics
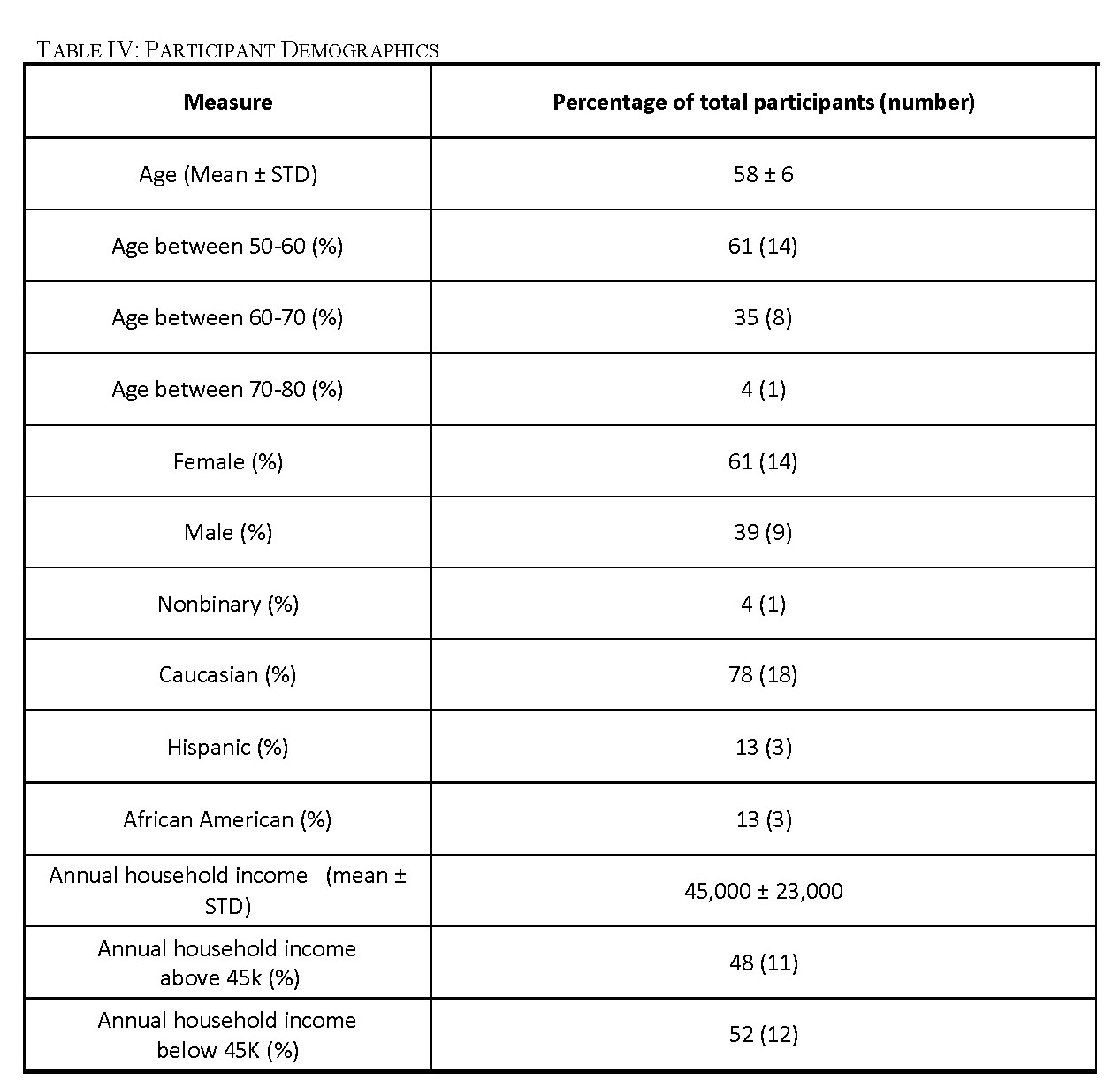
Through our recruitment efforts, we recruited 23 participants for four focus groups (Table IV). Our first focus group consisted of eight participants, all with documented histories of smoking, including six men and two women. This focus group included three minority participants with one individual who self-identified as African American and two individuals who self-identified as Hispanic. Our second focus group had seven participants, all with documented histories of smoking, including one nonbinary individual and six women. There were two individuals who self-identified as African American in the second focus group. Two women that met our criteria participated in our third focus group. Both participants identified as Caucasian. We had six participants in our fourth focus group including four women and two men. This focus group included two minority participants who both self-identified as Hispanic.
C. Findings from Focus Group Analysis
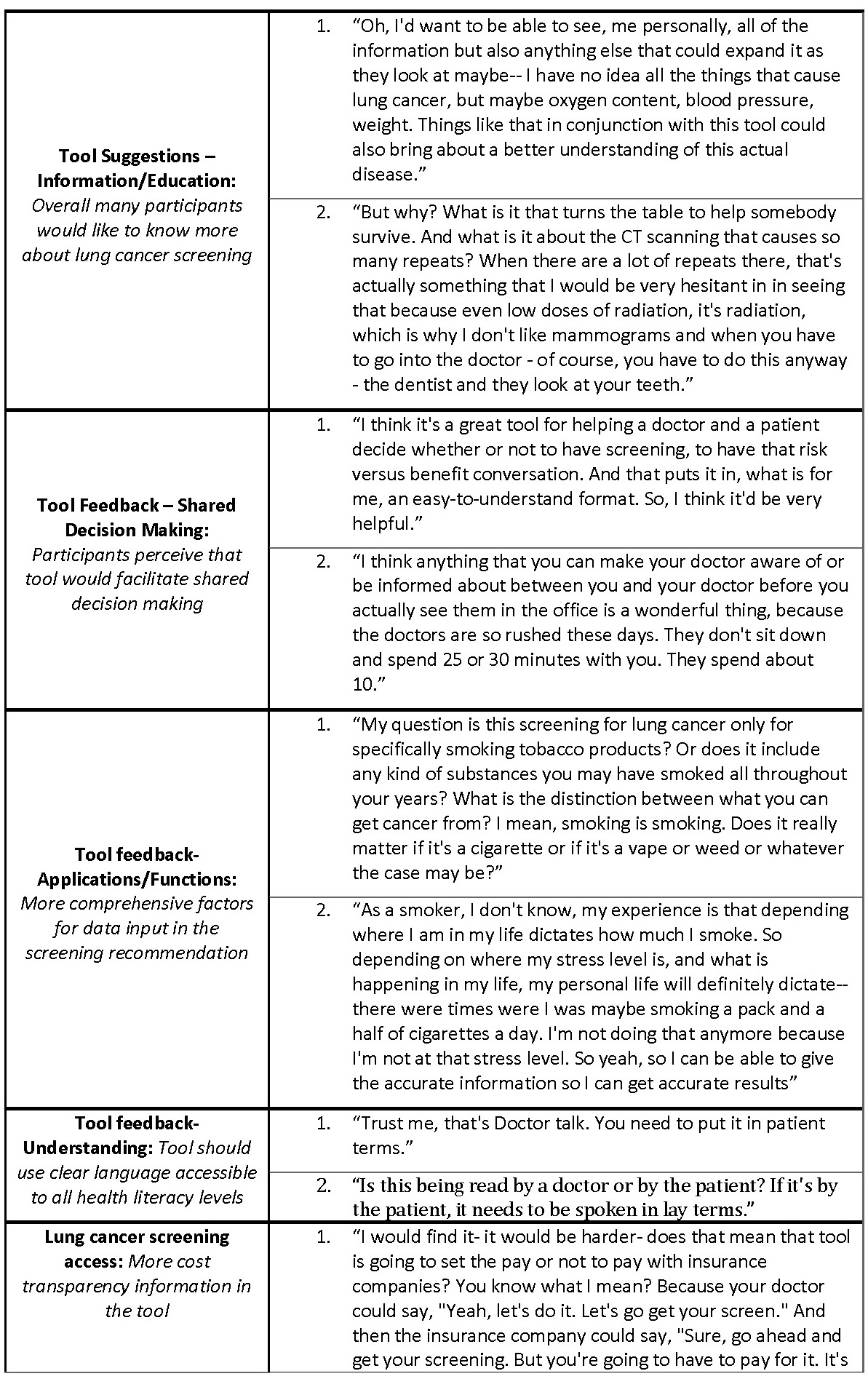
The responses to our focus group questions were coded using Dedoose software for aggregation and analysis. The general findings including the user needs, the facilitators, and barriers to tool use of the perceived tool were extracted from this analysis (Table V).
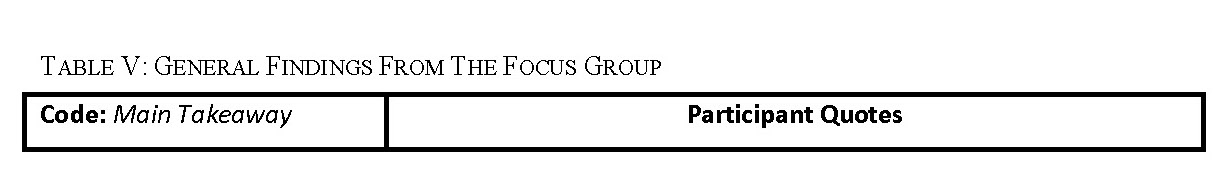
The focus group coding identified user needs relevant to developing our proposed interface. Feedback commonly given related to shared decision making was that the tool would facilitate shared decision making between the participants and their providers. In addition, participants did not always understand clearly the that the tool was meant to be used in the context of shared decision making in a provider visit. The two common misconceptions that participants had related to SDM was that the tool would make a screening decision for them, or the tool was meant to be used by a patient alone and wouldn’t be followed by a visit with their provider.
Focus group participants want to be better educated about lung cancer screening. Participants want to see the tool include more general information about lung cancer screening, a common takeaway from the excepts coded into ‘Tool Suggestions – Information/Education’. Some specific information that participants want to see includes a glossary of terms related to screening, information about how the timing of diagnosis influences lung cancer survival rate, and a basic overview the low-dose CT screening lung cancer diagnostic process.
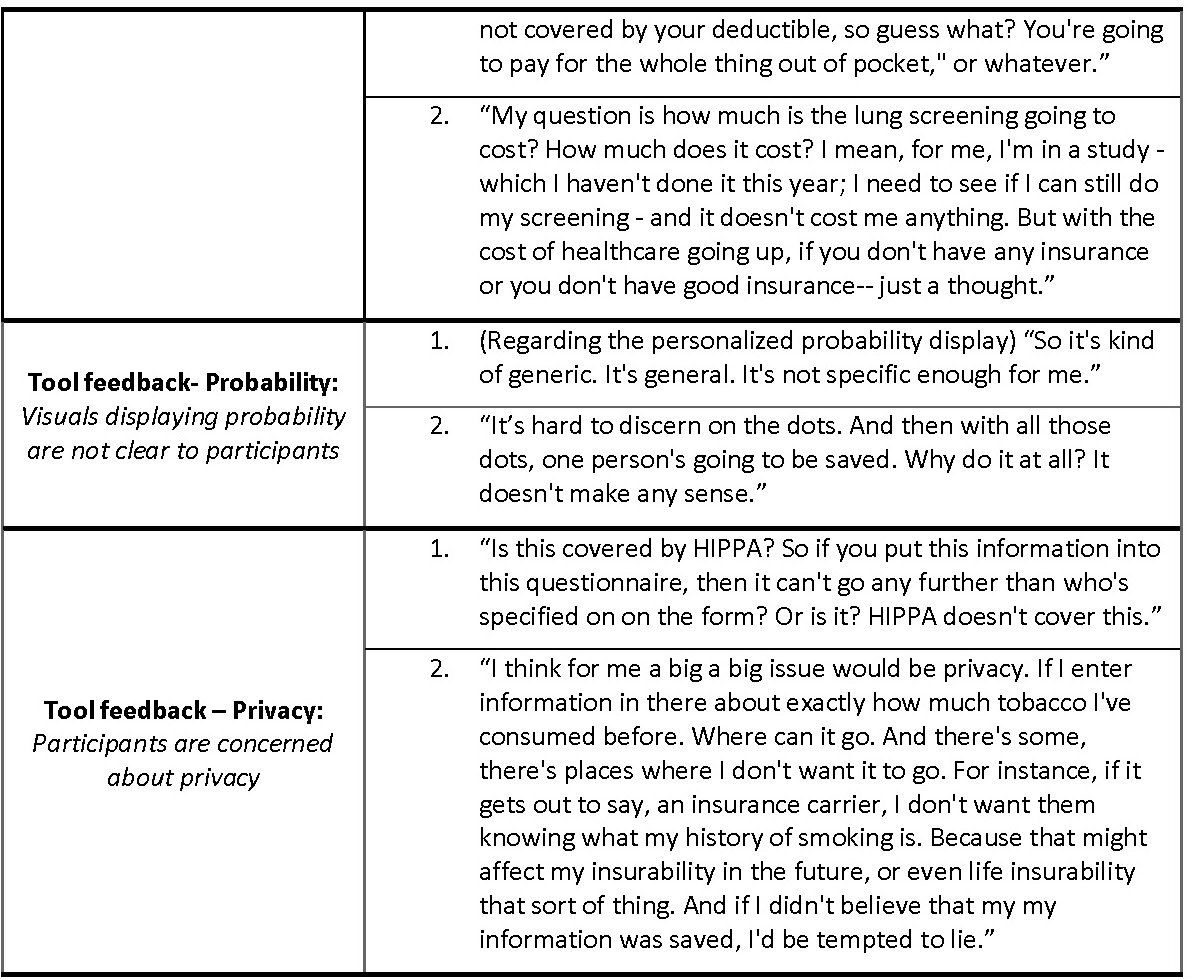
One common takeaway was that the tool would be more useful if the recommendation for screening was more comprehensive and included factors such as other substances used by the patient aside from tobacco, allowed for periods of non-smoking in between smoking, and took into account family history. Excerpts related to this idea were coded into ‘Tool feedback- Applications/Functions’. Participants also suggested that they would like to be able to modify the input data to reflect theoretical scenarios and see how that might change their risk for getting lung cancer. In addition, in our ‘Tool feedback- Understanding’ code, participants expressed a need for the language in the tool to be clear and accessible to all health literacy levels to make the perceived tool easy to use. Some terms that were not understood by participants included ‘pack-years’, ‘screening’ in the context of the tool, ‘quintile’, and ‘eligibility’ in the context of LCT screening. Potential users perceived that they would want information related to insurance coverage and cost of LCT screening on the tool. Many participants did not understand the relationship between the recommendation given in the tool and their access to insurance coverage for lung cancer screening (Table V: quote 1 and 2, ‘Lung cancer screening access’).
The probability conveyed on the tool in the dot display (Fig. 3) was overwhelmingly perceived as unclear and confusing to participants and commonly evoked feelings of fear regarding lung cancer (‘Tool feedback – Probability’). Participants frequently did not understand that the display was personalized to their risk for lung cancer (Table V: quote 1, ‘Tool feedback – Probability’). Lastly, many participants expressed concerns about their privacy related to their electronic health data as coded in ‘Tool feedback – Privacy’. Participants were especially concerned about how or if this information would be accessible to insurers or employers. The most common suggestions coded in these excepts was to make it clear who has access to the data entered on the tool and what measures were taken to protect it (Table V: quote 2, ‘Tool feedback – Privacy’ quote 2).
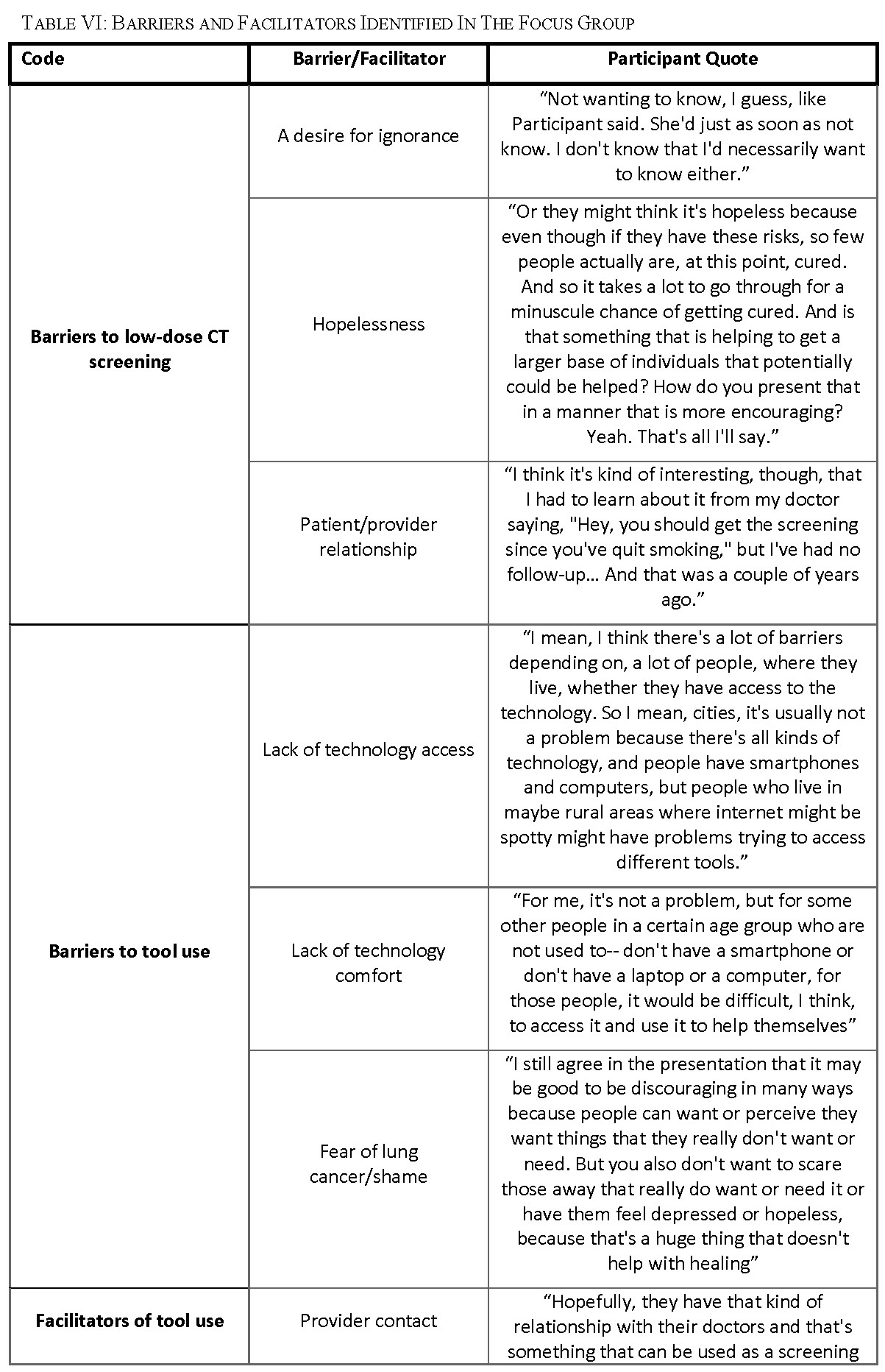
Barriers to low-dose CT screening for lung cancer were identified during the focus group (Table VI). Some participants expressed that they did not want to know if they had lung cancer or not; because they were not willing to change their behaviors regardless of the result (Table VI: ‘Barriers to low-dose CT screening – A desire for ignorance’). Others shared that they feel powerless to do anything about their risk for lung cancer and had no hope of improving their lung health (Table VI: ‘Barriers to low-dose CT screening – Hopelessness’). Feedback given related to this motif was to include in the tool more encouraging messaging on the tool and emphasizing how screening can help a patient take control of their own lung health. One participant said, “Some sort of ending on an encouraging note, because obviously, if somebody is looking at that, doing the tool, they may have indicators that they think that they could be eligible for lung cancer because they smoked … If you’re taking care of your health, you need to be reinforced with that, with the tools.” Another added that, “I think there needs to be some layer of hope in there too, that the earlier detection can make a difference.” Participants identified that in some situations their patient to provider relationship was a barrier to getting screened (Table VI: ‘Barriers to low-dose CT 24
screening – Patient/provider relationship’). Some reasons given for that were that their provider was not proactive about screening (Table VI: quote, ‘Barriers to low-dose CT screening – Negative patient/provider relationship’), they did not feel comfortable bringing it up with their provider or they are not honest with their provider.
Barriers to tool use were also extracted from the focus group (Table VI). This included a lack of access to appropriate technology use for various reasons including disability, physical barriers (i.e. rural or underserved populations) and language barriers (Table VI: ‘Barriers tool use– Lack of technology access’). Another barrier discussed is lack of technology comfort or computer illiteracy. The mean age of participants is 58 and 60% of our participants were between 50 and 60 which means that our participants are younger on average than the tool’s target population (50 to 80). So, the technology comfort barrier is likely even more prevalent among our general target user population than our results reflect. Lastly, participants identified fear and shame as a reason why they or others like them would not want to have a SDM process with their provider. In potentially related excerpts from the focus groups, stigma around smoking was a common topic and some participants even shared that they felt like, “second-class citizens” because they were smokers and that “smoking has become hush-hush. You don’t tell people [that you are a smoker].”
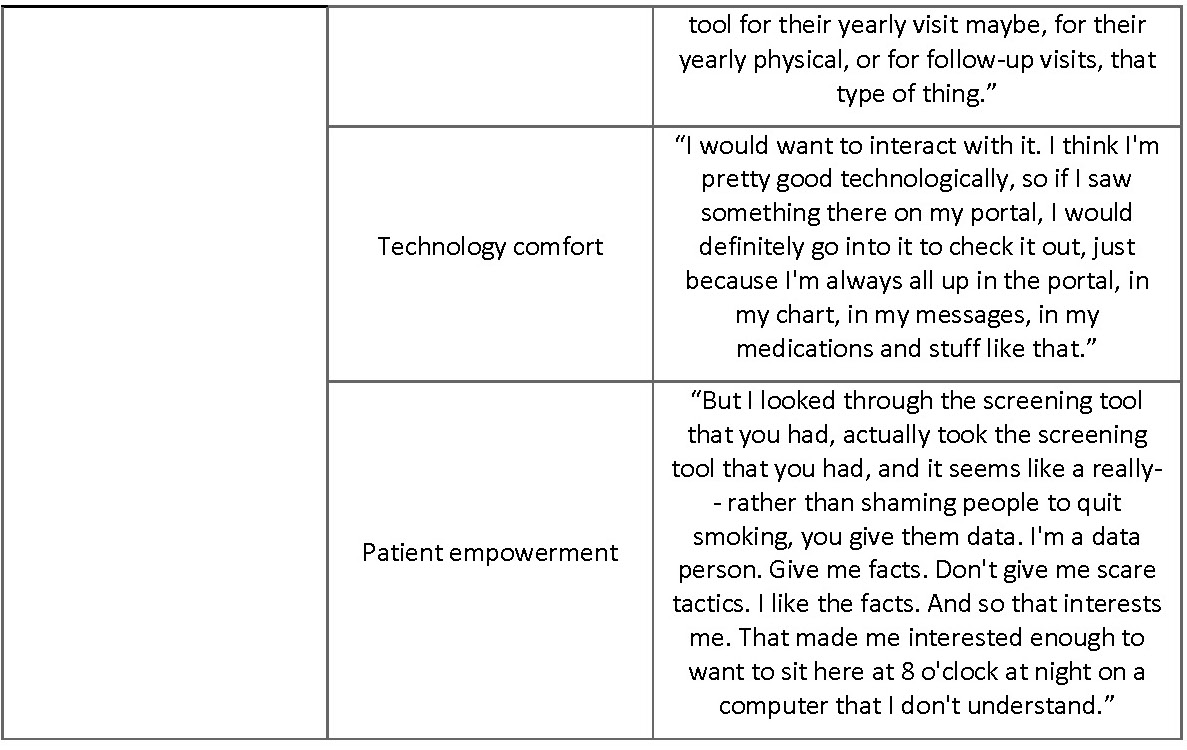
Facilitators for tool use were also coded in our analysis and topics including contact with a provider, technology comfort, and patient empowerment were all associated with perceived usefulness and ease of use of the tool (Table VI). Having regular contact with a primary care provider was perceived as a facilitator to tool use. Participants who felt empowered as a patient and wanted to be better informed often mentioned that they would use the tool, in contrast to participants who did not want to be better informed about their lung health or felt powerless to change their own lung health.
D. Post-Focus Group Survey Data
All 23 focus group participants answered all questions. Overall, the survey data showed that a patient-facing version of ScreenLC is perceived to be useful and relatively easy to use (Table VII, Fig. 4 & Fig. 5).
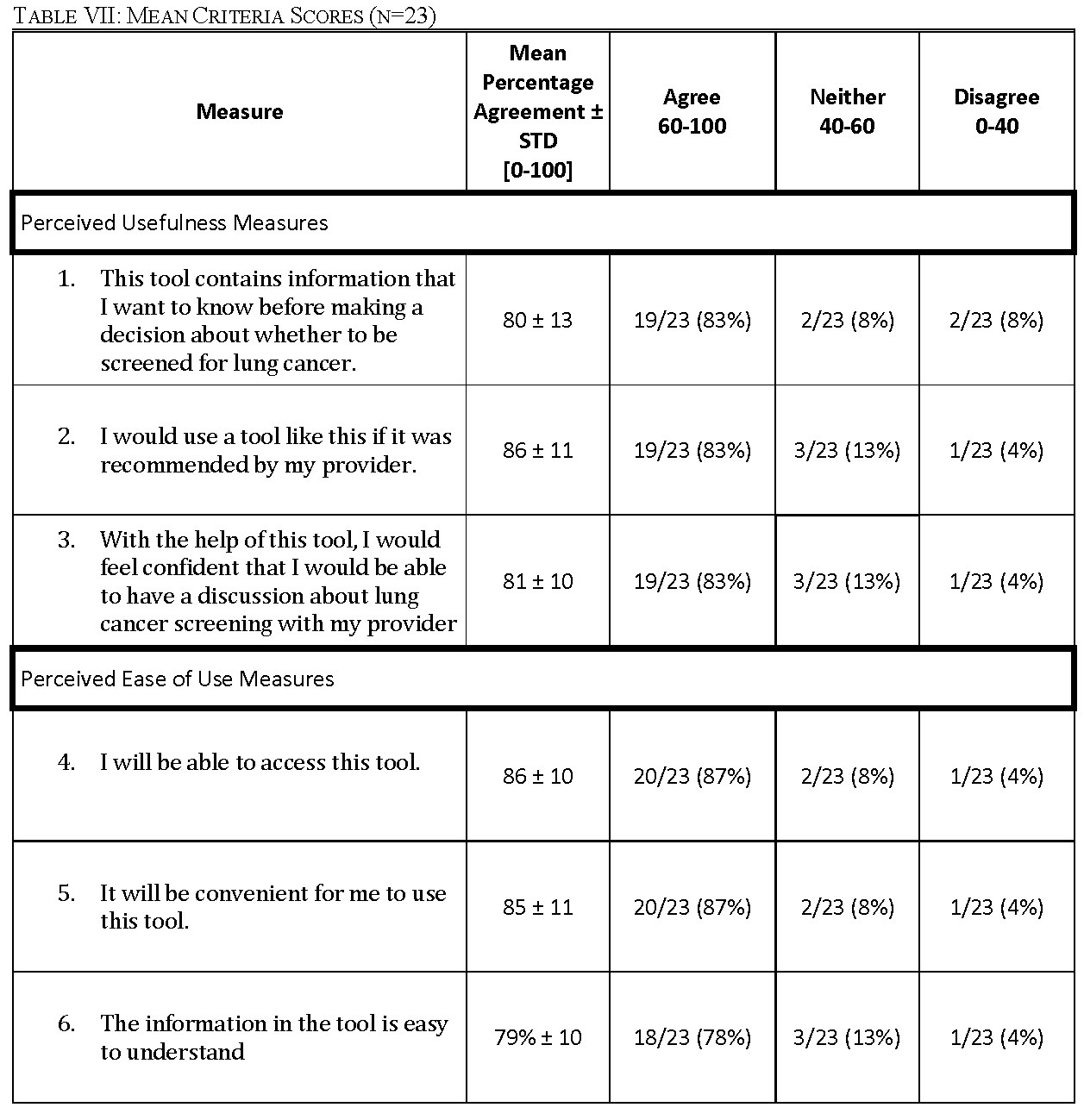
For each measure of perceived usefulness and perceived ease of use, no more than two participants disagreed with the measure (Table VII). For each question, the mean percentage agreement is over 80% aside from measure 6, with an agreement of 79%. This shows that the participants generally strongly agreed with the statements used to measure PU and PEU. The number of participants who ‘agree’, ‘neither agree nor disagree’ and ‘disagree’ with each measure is listed in Table VII. For each measure, no more than two participants disagreed with each statement.
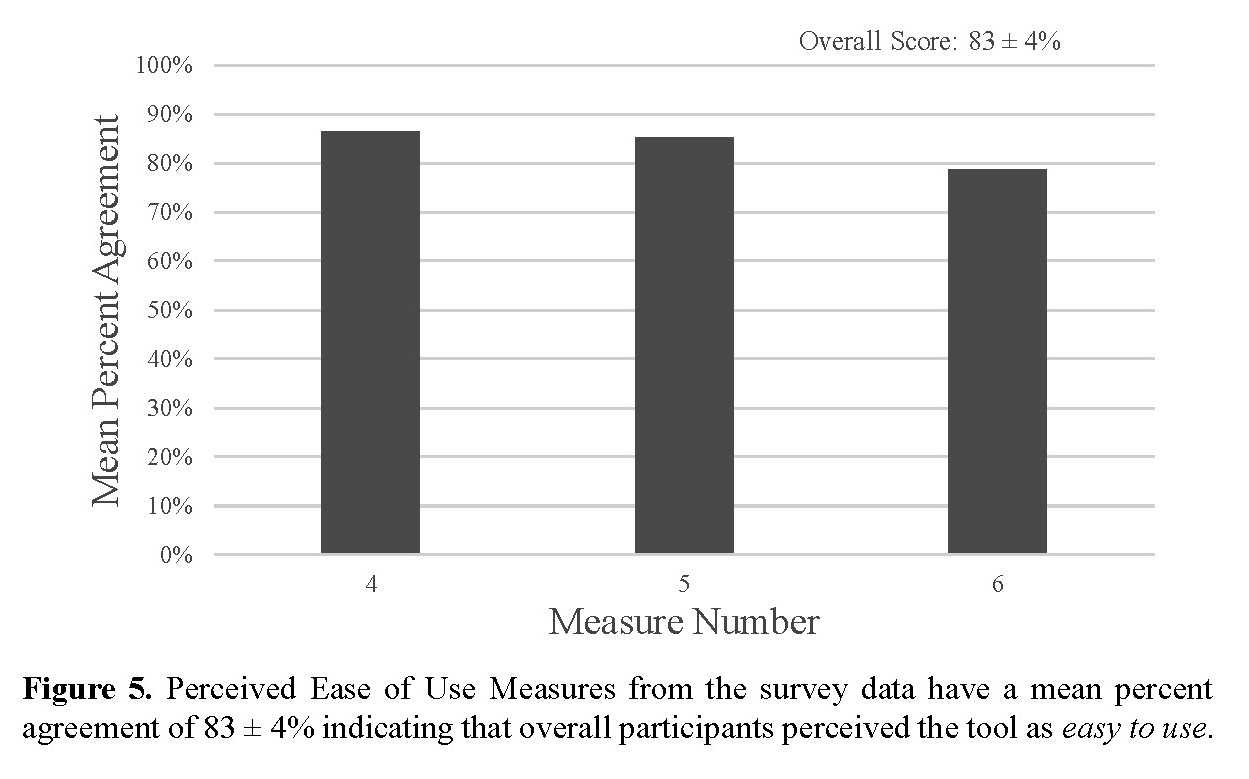
The overall score for the application’s usefulness as perceived by the participants was 82 ± 3 percent, which shows that the application is perceived to be useful (Fig. 4). The overall score for the ease of use of the tool as perceived by the participants was 83 ± 4 percent which shows that the application is perceived to be easy to use (Fig. 5).
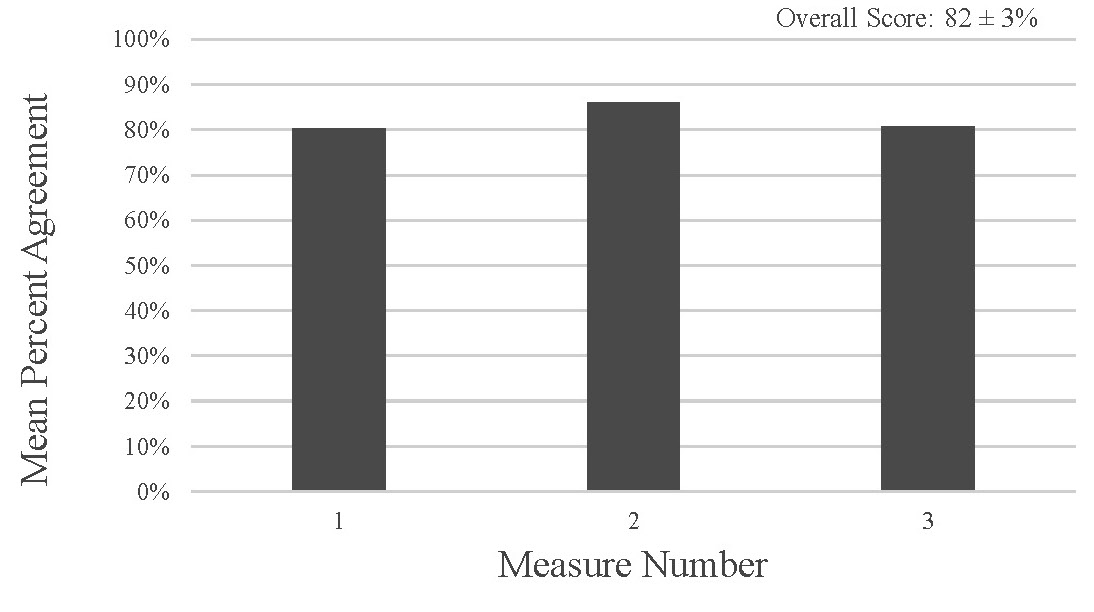
Figure 4. Perceived Usefulness Measures from the survey data have a mean percent agreement of 82 ± 3% indicating that overall participants perceived the tool as useful
V. DISCUSSION
Lung cancer should not take as many lives as it does in the United States, given the availability of modern, highly accurate lung cancer screening technology. However, this technology is not used by all the patients who could benefit from it. This disconnect between the number of patients who are screened and those who should be screened could be primarily due to a lack of patient knowledge [3]. To better inform patients about the need for screening, a patient-facing SDM tool needs to be developed. A few studies show that decision aids increase patient knowledge about lung cancer and a participant’s willingness to be screened when eligible [14]-[16]. However, the literature has not established the desired user experience of a web-based decision aid. Through a needs assessment for perceived usefulness and ease of use, we found a number of recommendations that should be incorporated into future tools.
Low-income populations in the United States are the least health literate of all income groups [49]. As shown in Table IV, our focus group participants had a mean household income of $45,000 which is considered low-income in the United States and approximately half of our participants are in the low-income bracket [35]. This will allow us to incorporate feedback from the population for which this tool is most needed. While the representation of minority groups in our focus groups was not as large as we wanted, we did have both African American and Hispanic participants. The mean age of our participants was 58 which may mean that our focus group participants are more health literate than our average target user (Table IV) [49].
Our focus group identified that participants wanted clearer visuals, low-dose CT screening education, and insight into how their data would be used (Table V). Many participants requested clarity on how the prediction calculator’s data are used and how the prediction was calculated and wanted a more comprehensive screening recommendation. Additionally, participants petitioned for more advice concerning the outcomes of their screening options, i.e., they wanted to know how the timing of the intervention would impact their health. We identified that participants preferred this information to be presented in clear visuals. The most effective presentation mode was determined to be simple graphs and scales. Addition thematic analysis is being conducted for further analysis.
From our analysis, stigma around smoking could be related to the shame and fear that prevents patients from engaging in shared decision making (Table VI). In past research, this has been identified as a barrier to successful lung cancer screening [37]. Depending on the patient and provider relationship, contact with the provider can serve as a facilitator or a barrier for engaging in shared decision making. Additional research is required to understand this relationship and solutions to overcoming this barrier.
We determined that our proposed lung-cancer screening SDM tool is useful and easy to use from the post-focus group survey (Fig. 4 & Fig. 5). This suggests that prospective users will most likely be willing and able to use the tool if it is provided. In the post-focus group survey, participants scored each measure to the extent to which they agree with the measure (Table VII). Participants scored how much they agreed with the statement, ‘This tool contains information that I want to know before I make decision about lung cancer with my provider’, as a measure of how useful the tool was perceived to be. The average score was 80 ± 13% indicating general agreement with that statement and the largest standard deviation out of all the measures by 2% (Table VII). This result is consistent with the focus group analysis in which participants had a wide variance in how useful they perceived the tool to be. Given feedback from the focus groups about how to include more patient specific information, we would expect this score to increase for the actual tool in which the results of this research would be incorporated. The statement ‘I would use a tool like this if it was recommended by my provider’ got an average score of 86 ± 11% (Table VII). The next measure of usability scored was ‘With the help of this tool, I would feel confident that I would be able to have a discussion about lung cancer screening with my provider’ got an average score of 81 ± 10% (Table VII). This is third lowest score out of all the measures. This score could be related to the stigma identified as a barrier to screening in our research as well as others and further research is necessary to understand this stigma and how to overcome it [37]. Our first measure of the tool’s perceived ease of use is how respondents scored the statement, “I will be able to access this tool.” The average score was 86 ± 11% (Table VII). Next, the statement, “It will be convenient for me to use this tool,” was scored similarly with an average score of 85 ± 11% (Table VII). Lastly, the statement, “the information in this tool is easy to understand,” had the lowest score with an average score of 79 ± 10% (Table VII). This is consistent with the focus group and would be expected to increase following incorporation of the focus group feedback. Our quantitative results indicate that continued development of a patient-facing tool would prove an effective use of resources and time to work towards the goal of empowering patients to make informed decisions regarding their lung health. Adaptation from ScreenLC will decrease the recourses and time required for the development of the tool.
From the results, we suggest the following recommendations for the next version of the tool, adapted from ScreenLC, for patient access at all times:
1. Providers engaging with patients prior to using tool
2. Clear language and messaging on how to use the tool in a shared decision-making process
3. Additional education materials including:
a. How the screening recommendation is determined
b. Basic information about what lung cancer screening is and the diagnostic process
4. Simple visuals and statistics to covey the personalized lung cancer risk
Our results and these recommendations informed by our results are in line with what little has been established regarding the user needs for a lung cancer screening SDM tool. Specifically, the need for more information and clear language [17], [18], [52].
The privacy concerns could be addressed with a SMART (SMART Health IT, Boston Children’s Hospital, Boston, MA) authorization flow meaning that all of the data for the tool would be accessed from the user’s device and no backend server would have access to the token that authorizes use of the EHR. The user would be made aware of this through a simple information blurb next to the data entry stating that their information is protected and who can access it.
One strength of our potential tool is that through this analysis, the voice of patients will be taken into account during development. When creating a shared decision-making tool, too often, patients are not included in the process of determining the user requirements and specifications for the tool being developed. In most cases, this lack of insight would decrease the usefulness and ease of use of the tool by patients [39]. Development and testing with patient input is recommended for effective SDM tools [39], [50]. Currently, in lung cancer screening SDM, educational tools or materials have not been utilized effectively and there is a need for highly useable and easy to use tools [48], [51]. In addition, it has been shown that barriers to lung cancer screening should be taken into account when developing decision tools for screening [37]. Many of our findings were not anticipated by our team and will shape our design in significant ways to improve the usability of the end product.
SDM tools in the context of lung cancer screening are shown to be effective in empowering patients when developed to be acceptable and feasible to patients [17], [52]. When patients are empowered with personalized information, screening rates are expected to increase but the extent of this increase is unknown and not agreed upon [9], [15], [53]. Further research is needed regarding the impact of SDM tools on screening rates.
The major limitation of the presented work was the small sample size. With a sample size of 23 we do not have the statistical power for the study to be representative of the whole target
population but rather just a sample and further research should be done to generalize these results. Additionally, our study aimed to examine the user needs of a specific population of patients. However, informational needs were only gathered from human subjects that could be contacted by our team, which may have excluded target users that our team could not contact. In addition, only English-speaking participants were included in the focus groups. Lastly, there may be a gap between the perceived usefulness and perceived ease of use versus the actual usefulness and ease of use, or the usefulness and ease of use of the developed application.
In conclusion, our study supports the case for the development of shared decision-making tools to assist patients in making potentially life-saving low-dose CT lung cancer screening decisions as patients are interested and perceive the SDM tool to be useful. The methods of this study ensure that patient feedback is incorporated into the final SDM tool. Based on feedback from patients, some recommendations include data sharing should be disclosed, simple visuals or percentages should be used to display probability, and the context of the tool in the SDM process should be explicit on the tool. Much work still needs to be done to verify and validate such tools and establish a dissemination strategy to ensure it reaches the patients who need it the most. The intention is that through the development of web-based, mobile-accessible SDM tools, we should be able to increase the utilization of low-dose CT screening by eligible patients and ultimately decrease lung cancer mortality.


VII. ACKNOWLEDGEMENTS
I would like to thank Dr. Kimberly Kaphingst (University of Utah) and the Community Collaboration and Engagement Team (CCET) at the University of Utah’s Center for Clinical and Translational Science for their collaboration efforts.
VIII. REFERENCES
[1] R. L. Siegel, K. D. Miller, and A. Jemal, “Cancer statistics, 2020,” CA Cancer J Clin, vol. 70, no. 1, pp. 7–30, Jan. 2020, doi: 10.3322/caac.21590.
[2] A. L. Association, “New Report: Critically Low Lung Cancer Screening Rates Reveal Opportunity to Save More Lives.” https://www.lung.org/media/press-releases/state-of-lung-cancer-2022 (accessed Jan. 11, 2023).
[3] E. Sosa et al., “Racial and Socioeconomic Disparities in Lung Cancer Screening in the US: A Systematic Review,” CA Cancer J Clin, vol. 71, no. 4, pp. 299–314, Jul. 2021, doi: 10.3322/caac.21671.
[4] A. C. Melzer, S. E. Golden, S. S. Ono, S. Datta, K. Crothers, and C. G. Slatore, “What Exactly Is Shared Decision-Making? A Qualitative Study of Shared Decision-Making in Lung Cancer Screening,” J Gen Intern Med, vol. 35, no. 2, pp. 546–553, Feb. 2020, doi: 10.1007/s11606-019-05516-3.
[5] R. Wender et al., “American Cancer Society lung cancer screening guidelines,” CA: A Cancer Journal for Clinicians, vol. 63, no. 2, pp. 106–117, 2013, doi: 10.3322/caac.21172.
[6] “Definition of shared decision making – NCI Dictionary of Cancer Terms – NCI,” Feb. 02, 2011. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/shared-decision-making (accessed May 03, 2023).
[7] Y.-C. T. Shih, Y. Xu, L. M. Lowenstein, and R. J. Volk, “Implementation of Shared Decision Making for Lung Cancer Screening Among the Privately Insured Nonelderly,” MDM Policy & Practice, vol. 6, no. 1, p. 2381468320984773, Jan. 2021, doi: 10.1177/2381468320984773.
[8] G. Elwyn et al., “Shared Decision Making: A Model for Clinical Practice,” J GEN INTERN MED, vol. 27, no. 10, pp. 1361–1367, Oct. 2012, doi: 10.1007/s11606-012-2077-6.
[9] A. T. Brenner et al., “Evaluating Shared Decision Making for Lung Cancer Screening,” JAMA Internal Medicine, vol. 178, no. 10, pp. 1311–1316, Oct. 2018, doi: 10.1001/jamainternmed.2018.3054.
[10] A. M. O’Connor et al., “Toward The ‘Tipping Point’: Decision Aids And Informed Patient Choice,” Health Affairs, vol. 26, no. 3, pp. 716–725, May 2007, doi: 10.1377/hlthaff.26.3.716.
[11] C. B. [D-N.-15 Rep. Rangel, “Text – H.R.3590 – 111th Congress (2009-2010): Patient Protection and Affordable Care Act,” Mar. 23, 2010. http://www.congress.gov/ (accessed Mar. 31, 2023).
[12] R. A. Smith et al., “Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening,” CA: A Cancer Journal for Clinicians, vol. 68, no. 4, pp. 297–316, 2018, doi: 10.3322/caac.21446.
[13] “Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology in: Journal of the National Comprehensive Cancer Network Volume 16 Issue 4 (2018).” https://jnccn.org/view/journals/jnccn/16/4/article-p412.xml?rskey=gCbwL3&result=1318&utm_source=TrendMD&utm_medium=cpc&utm_campaign=JNCCN_TrendMD_1 (accessed May 03, 2023).
[14] P. J. Mazzone et al., “Screening for Lung Cancer: CHEST Guideline and Expert Panel Report,” Chest, vol. 153, no. 4, pp. 954–985, Apr. 2018, doi: 10.1016/j.chest.2018.01.016.
[15] A. C. Melzer, S. E. Golden, S. S. Ono, S. Datta, K. Crothers, and C. G. Slatore, “What Exactly Is Shared Decision-Making? A Qualitative Study of Shared Decision-Making in Lung Cancer Screening,” J Gen Intern Med, vol. 35, no. 2, pp. 546–553, Feb. 2020, doi: 10.1007/s11606-019-05516-3.
[16] G. Elwyn et al., “Developing a quality criteria framework for patient decision aids: online international Delphi consensus process,” BMJ, vol. 333, no. 7565, p. 417, Aug. 2006, doi: 10.1136/bmj.38926.629329.AE.
[17] K. K. McDonnell et al., “Developing and testing a brief clinic-based lung cancer screening decision aid for primary care settings,” Health Expectations, vol. 21, no. 4, pp. 796–804, 2018, doi: 10.1111/hex.12675.
[18] D. S. Reuland, L. Cubillos, A. T. Brenner, R. P. Harris, B. Minish, and M. P. Pignone, “A pre-post study testing a lung cancer screening decision aid in primary care,” BMC Medical Informatics and Decision Making, vol. 18, no. 1, p. 5, Jan. 2018, doi: 10.1186/s12911-018-0582-1.
[19] O. Karnieli-Miller and Z. Eisikovits, “Physician as partner or salesman? Shared decision-making in real-time encounters,” Social Science & Medicine, vol. 69, no. 1, pp. 1–8, Jul. 2009, doi: 10.1016/j.socscimed.2009.04.030.
[20] F. C. Detterbeck, P. J. Mazzone, D. P. Naidich, and P. B. Bach, “Screening for Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines,” Chest, vol. 143, no. 5, Supplement, pp. e78S-e92S, May 2013, doi: 10.1378/chest.12-2350.
[21] R. J. Volk and D. Stacey, “Ensuring High-Quality Shared Decision-making for Lung Cancer Screening,” JAMA Oncology, vol. 8, no. 11, pp. 1561–1562, Nov. 2022, doi: 10.1001/jamaoncol.2022.3766.
[22] US Preventive Services Task Force et al., “Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement,” JAMA, vol. 325, no. 10, pp. 962–970, Mar. 2021, doi: 10.1001/jama.2021.1117.
[23] “National Lung Screening Trial: Questions and Answers – NCI,” Sep. 18, 2002. https://www.cancer.gov/types/lung/research/nlst-qa (accessed Mar. 31, 2023).
[24] M. B. Schabath and M. L. Cote, “Cancer Progress and Priorities: Lung Cancer,” Cancer Epidemiol Biomarkers Prev, vol. 28, no. 10, pp. 1563–1579, Oct. 2019, doi: 10.1158/1055-9965.EPI-19-0221.
[25] “Cancer Prevention & Early Detection| American Cancer Society.” https://www.cancer.org/research/cancer-facts-statistics/cancer-prevention-early-detection.html (accessed Mar. 31, 2023).
[26] E. F. Patz Jr et al., “Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer,” JAMA Internal Medicine, vol. 174, no. 2, pp. 269–274, Feb. 2014, doi: 10.1001/jamainternmed.2013.12738.
[27] J. M. Albert, “Radiation Risk From CT: Implications for Cancer Screening,” American Journal of Roentgenology, vol. 201, no. 1, pp. W81–W87, Jul. 2013, doi: 10.2214/AJR.12.9226.
[28] D. A. Pierce and D. L. Preston, “Radiation-Related Cancer Risks at Low Doses among Atomic Bomb Survivors,” Radiation Research, vol. 154, no. 2, pp. 178–186, Aug. 2000, doi: 10.1667/0033-7587(2000)154[0178:RRCRAL]2.0.CO;2.
[29] P. B. Bach et al., “Benefits and Harms of CT Screening for Lung Cancer: A Systematic Review,” JAMA, vol. 307, no. 22, pp. 2418–2429, Jun. 2012, doi: 10.1001/jama.2012.5521.
[30] M. Warschauer, Technology and Social Inclusion: Rethinking the Digital Divide. MIT Press, 2004.
[31] F. D. Davis, “Perceived Usefulness, Perceived Ease of Use, and User Acceptance of Information Technology,” MIS Quarterly, vol. 13, no. 3, pp. 319–340, 1989, doi: 10.2307/249008.
[32] “Making health communication programs work.” https://stacks.cdc.gov/view/cdc/24017 (accessed Mar. 31, 2023).
[33] J. Francis et al., “Constructing questionnaires based on the theory of planned behaviour: A manual for Health Services Researchers.,” Quality of life and management of living resources; Centre for Health Services Research, 2004, Accessed: Mar. 31, 2023. [Online]. Available: https://abdn.pure.elsevier.com/en/publications/constructing-questionnaires-based-on-the-theory-of-planned-behavi
[34] “Comparison of Four TAM Item Formats: Effect of Response Option Labels and Order – JUX,” JUX – The Journal of User Experience, Aug. 30, 2019. https://uxpajournal.org/tam-formats-effect-response-labels-order/ (accessed Mar. 31, 2023).
[35] M. Barrera, F. G. Castro, L. A. Strycker, and D. J. Toobert, “Cultural adaptations of behavioral health interventions: a progress report,” J Consult Clin Psychol, vol. 81, no. 2, pp. 196–205, Apr. 2013, doi: 10.1037/a0027085.
[36] A. K. Borondy Kitts, “The Patient Perspective on Lung Cancer Screening and Health Disparities,” J Am Coll Radiol, vol. 16, no. 4 Pt B, pp. 601–606, Apr. 2019, doi: 10.1016/j.jacr.2018.12.028.
[37] L. Carter-Harris and M. K. Gould, “Multilevel Barriers to the Successful Implementation of Lung Cancer Screening: Why Does It Have to Be So Hard?,” Annals ATS, vol. 14, no. 8, pp. 1261–1265, Aug. 2017, doi: 10.1513/AnnalsATS.201703-204PS.
[38] F. Cartujano-Barrera et al., “Feasibility and Acceptability of a Culturally- and Linguistically-Adapted Smoking Cessation Text Messaging Intervention for Latino Smokers,” Front Public Health, vol. 8, p. 269, Jun. 2020, doi: 10.3389/fpubh.2020.00269.
[39] V. Chenel, W. B. Mortenson, M. Guay, J. W. Jutai, and C. Auger, “Cultural adaptation and validation of patient decision aids: a scoping review,” Patient Prefer Adherence, vol. 12, pp. 321–332, 2018, doi: 10.2147/PPA.S151833.
[40] J. S. Choi et al., “Cultural Adaptation of a Community-Based Hearing Health Intervention for Korean American Older Adults with Hearing Loss,” J Cross Cult Gerontol, vol. 34, no. 3, pp. 223–243, Sep. 2019, doi: 10.1007/s10823-019-09376-6.
[41] C. Escoffery et al., “A systematic review of adaptations of evidence-based public health interventions globally,” Implementation Science, vol. 13, no. 1, p. 125, Sep. 2018, doi: 10.1186/s13012-018-0815-9.
[42] C. J. Etzel et al., “Development and validation of a lung cancer risk prediction model for African-Americans,” Cancer Prev Res (Phila), vol. 1, no. 4, pp. 255–265, Sep. 2008, doi: 10.1158/1940-6207.CAPR-08-0082.
[43] K. L. Kumpfer, M. Pinyuchon, A. Teixeira de Melo, and H. O. Whiteside, “Cultural adaptation process for international dissemination of the strengthening families program,” Eval Health Prof, vol. 31, no. 2, pp. 226–239, Jun. 2008, doi: 10.1177/0163278708315926.
[44] F. F. Marsiglia and J. M. Booth, “Cultural Adaptation of Interventions in Real Practice Settings,” Res Soc Work Pract, vol. 25, no. 4, pp. 423–432, Jul. 2015, doi: 10.1177/1049731514535989.
[45] A. C. Melzer, S. E. Golden, S. S. Ono, S. Datta, K. Crothers, and C. G. Slatore, “What Exactly Is Shared Decision-Making? A Qualitative Study of Shared Decision-Making in Lung Cancer Screening,” J Gen Intern Med, vol. 35, no. 2, pp. 546–553, Feb. 2020, doi: 10.1007/s11606-019-05516-3.
[46] K. Resnicow, R. Soler, R. L. Braithwaite, J. S. Ahluwalia, and J. Butler, “Cultural sensitivity in substance use prevention,” Journal of Community Psychology, vol. 28, no. 3, pp. 271–290, 2000, doi: 10.1002/(SICI)1520-6629(200005)28:3<271::AID-JCOP4>3.0.CO;2-I.
[47] R. J. Volk et al., “Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography,” Preventive Medicine, vol. 62, pp. 60–63, May 2014, doi: 10.1016/j.ypmed.2014.02.006.
[48] G. X. Wang et al., “Barriers to Lung Cancer Screening Engagement from the Patient and Provider Perspective,” Radiology, vol. 290, no. 2, pp. 278–287, Feb. 2019, doi: 10.1148/radiol.2018180212.
[49] K. T. Hickey et al., “Low health literacy,” Nurse Pract, vol. 43, no. 8, pp. 49–55, Aug. 2018, doi: 10.1097/01.NPR.0000541468.54290.49.
[50] M. A. Smith, “The Role of Shared Decision Making in Patient-Centered Care and Orthopaedics,” Orthopaedic Nursing, vol. 35, no. 3, pp. 144–149, May 2016, doi: 10.1097/NOR.0000000000000243.
[51] S. P. E. Nishi et al., “Shared Decision-Making for Lung Cancer Screening: How Well Are We ‘Sharing’?,” Chest, vol. 160, no. 1, pp. 330–340, Jul. 2021, doi: 10.1016/j.chest.2021.01.041.
[52] R. J. Volk et al., “Effect of a Patient Decision Aid on Lung Cancer Screening Decision-Making by Persons Who Smoke: A Randomized Clinical Trial,” JAMA Network Open, vol. 3, no. 1, p. e1920362, Jan. 2020, doi: 10.1001/jamanetworkopen.2019.20362.
[53] M. I. Fukunaga et al., “Tools to Promote Shared Decision-Making in Lung Cancer Screening Using Low-Dose CT Scanning: A Systematic Review,” Chest, vol. 158, no. 6, pp. 2646–2657, Dec. 2020, doi: 10.1016/j.chest.2020.05.610.

