School of Medicine
53 Control of Isolation Induced Aggression through Activation of Medial Prefrontal Cortex Pyramidal Neurons
Jordyn Gagon; Rachel E. Gatlin (Neurobiology and Anatomy); and Moriel Zelikowksky (Neurobiology and Anatomy)
Faculty Mentor: Moriel Zelikowksky (School of Medicine, University of Utah)
Background
The effects of social isolation have recently been felt deeply across the globe as the world seemingly shut down in response to the novel Covid-19 pandemic. While social isolation is critical in slowing disease spread, social interaction is critical for human health. Social isolation has been linked to poor health, depression, and overall decline in well-being (Courtin and Knapp, 2015). The effects of social isolation have been seen before, as prison inmates who have been placed in solitary confinement are more likely to experience aggression, suicidal ideology, and irritability (Scharf Smith, 2006). Furthermore, isolated mice show increased aggression compared to group housed mice (Zelikowsky et. al., 2018).
The prefrontal cortex (PFC), which is involved in emotional and cognitive regulation, is a target area of the brain in controlling aggression. Damage to the PFC can result is severe emotional disruption, often leading to aggression. Patients with penetrating traumatic brain injury with lesions specific to PFC territories displayed the highest levels of aggression compared to patients with lesions in other parts of the brain. In a study examining patients with penetrating traumatic brain injury, patients with the highest levels of aggression primarily presented lesions in the PFC region (Pardini et. al., 2011). One historic example of this damage is Phineas Gage, a railroad worker who experienced an injury in which a rod penetrated his skull and into the PFC. Following the injury, Phineas Gage became angry and showed poor social judgement (Siever, 2008).
Previous work has established that medial prefrontal cortex (mPFC) pyramidal neurons project to multiple subcortical aggression centers (Biro et. al., 2018), and that inhibition of such neurons in mPFC reduces aggression (Takahashi et. al. 2014; Van Heukelum et. al, 2021). This work has led to a model of the mPFC controlling aggression through these pyramidal neurons in which activation of mPFC pyramidal neurons in aggressive mice reduces aggression and inhibition of mPFC pyramidal neurons seems to enhance aggression. Despite this, no work has focused on how this population of neurons may regulate social isolation-induced aggression nor have these neurons been studied in females. Here, we expand on previous work showing a link between decreased mPFC pyramidal neuron activity and aggression in males by examining whether activation of mPFC pyramidal neurons reduces aggression in socially isolated female mice. We hypothesize that activation of mPFC pyramidal neurons in socially isolated female animals will reduce aggressive behavior.
Methods
A Designer Receptor Exclusively Activated by Designer Drugs (DREADD)-mediated approach was taken to activate the mPFC pyramidal neurons. To this end we infused a virus encoding the excitatory DREADD, hM3D, fused to mCherry, under control of the CaMKII promoter into the mPFC of female C57Bl6/N mice (N=8). This will express the activating DREADD in the pyramidal neurons of the mPFC. A virus expressing mCherry without the hM3D DREADD under the control of the CaMKII promoter was used as a control (N=9). Following surgery, all mice were isolated (single housed) for four weeks to induce aggression. Each animal was then tested on the resident intruder assay in which a novel age and sex matched docile mouse is introduced into the home cage of the test animal. Each test animal was tested twice, once in which the DREADD ligand, Descholorclozapine (DCZ) administered via i.p, injection and once with vehicle solution, 48 hours apart. The viral conditions were counterbalanced such that half the animals in each viral condition (hM3D or mCherry control) received DCZ on the first test day and half received an injection of the vehicle on the first test day. High-speed video was recorded during the resident intruder assay, which was then used to score aggressive (attack) and social behaviors (face investigation, anogenital investigation, and body investigation) using Noldus Observer. Primarily, attack behavior was defined as the test animal tussling, biting, and kicking the intruder. Mounting is defined when the test animal is on top of the intruder, but not kicking or biting. Lastly, anogenital investigation is defined as the test animal sniffing the rear end of the intruder. Upon the conclusion of testing, all brains were extracted, sectioned, and imaged to confirm virus expression in the mPFC (Figure 1).
Results
DREADD-mediated activation of mPFC pyramidal neurons significantly decreased attack aggression (Repeated Measures ANOVA, p<0.05) (Figure 2). However, activation of mPFC pyramidal neurons had no effect on other social behaviors, including mounting (p=0.2079) (Figure 3) nor anogenital investigation (p=.9955) (Figure 4). Furthermore, there were no significant results within the control mCherry condition, suggesting that DCZ did not have any effect on behavior in the absence of the DREADD.
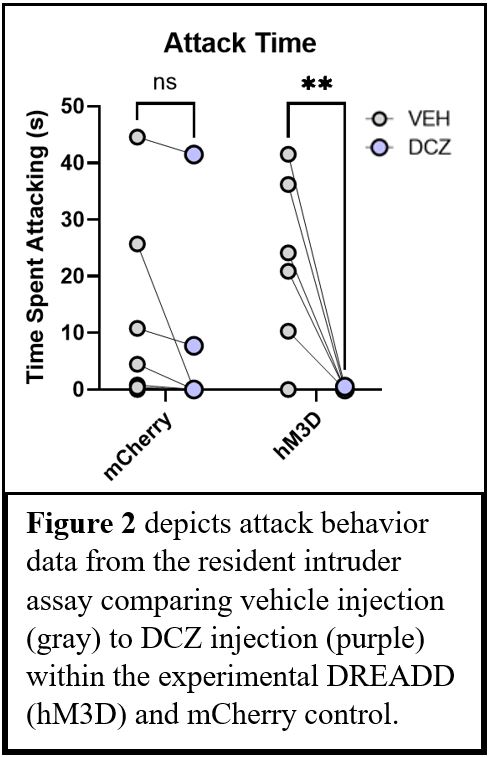
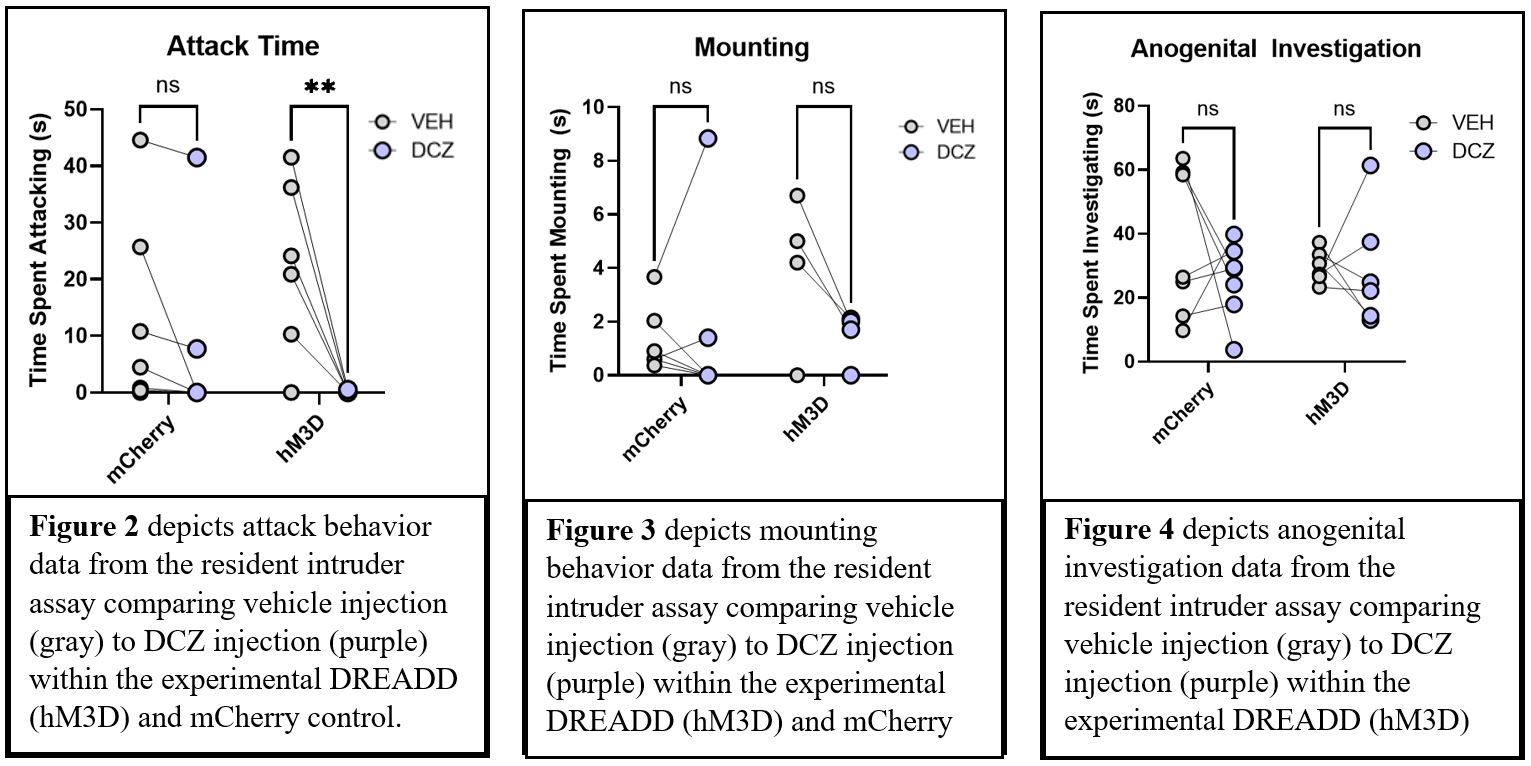
Conclusions
These results support the hypothesis that the mPFC plays an inhibitory role in aggressive behavior. Additionally, mPFC pyramidal neurons are essential to this inhibitory control. Activation of mPFC pyramidal neurons seems to exclusively alter aggression and does not affect other social behaviors, such as anogenital investigation. Next steps include activating mPFC pyramidal neurons in male mice to compare differences across sex.
Acknowledgements
This work was supported by the Office of Undergraduate Research through the University of Utah and the Rural and Underserved Utah Training Experience (RUUTE) Summer Undergraduate Research Experience (SURE) program through the University of Utah School of Medicine. A huge thank you to Dr. Zelikowsky, Rachel E. Gatlin, and the rest of the Zelikowsky lab for their help and mentorship throughout this project and my undergraduate research.
References
Biro, Laszlo, et al. “Task Division within the Prefrontal Cortex: Distinct Neuron Populations Selectively Control Different Aspects of Aggressive Behavior via the Hypothalamus.” The Journal of Neuroscience, vol. 38, no. 17, 2018, pp. 4065–4075., https://doi.org/10.1523/jneurosci.3234-17.2018.
Courtin, Emilie, and Martin Knapp. “Social Isolation, Loneliness and Health in Old Age: A Scoping Review.” Health & Social Care in the Community, vol. 25, no. 3, 2015, pp. 799–812., https://doi.org/10.1111/hsc.12311.
Pardini, M., et al. “Prefrontal Cortex Lesions and MAO-A Modulate Aggression in Penetrating Traumatic Brain Injury.” Neurology, vol. 76, no. 12, 2011, pp. 1038–1045., https://doi.org/10.1212/wnl.0b013e318211c33e.
Siever, Larry J. “Neurobiology of Aggression and Violence.” American Journal of Psychiatry, vol. 165, no. 4, 2008, pp. 429–442., https://doi.org/10.1176/appi.ajp.2008.07111774.
Smith, Peter Scharff. “The Effects of Solitary Confinement on Prison Inmates: A Brief History and Review of the Literature.” Crime and Justice, vol. 34, no. 1, 2006, pp. 441–528., https://doi.org/10.1086/500626.
Takahashi, Aki, et al. “Control of Intermale Aggression by Medial Prefrontal Cortex Activation in the Mouse.” PLoS ONE, vol. 9, no. 4, 2014, https://doi.org/10.1371/journal.pone.0094657.
Van Heukelum, Sabrina, et al. “A Central Role for Anterior Cingulate Cortex in the Control of Pathological Aggression.” Current Biology, vol. 31, no. 11, 2021, https://doi.org/10.1016/j.cub.2021.03.062.
Zelikowsky, Moriel, et al. “The Neuropeptide TAC2 Controls a Distributed Brain State Induced by Chronic Social Isolation Stress.” Cell, vol. 173, no. 5, 2018, https://doi.org/10.1016/j.cell.2018.03.037.

