College of Engineering
31 Electrical Impedance Dermography as a Biomarker for Non-Melanoma Skin Cancer
Elaine Wen Ying Wong; Benjamin Sanchez (Electrical and Computer Engineering); and Douglas Grossman (Huntsman Cancer Institute)
Faculty Mentor: Benjamin Sanchez (Electrical and Computer Engineering, University of Utah)
Abstract: Diagnosis of basal cell and squamous cell carcinoma subtypes is difficult due to being unable to know how deep the cancerous tissue goes. Therefore, different subtypes ideally require different types of biopsies, but due to being unable to visually see how deep the cancerous tissue goes, even expert dermatologists struggle to make this decision. Therefore, we hypothesize that using electrical impedance dermography can serve as a biomarker to distinguish these subtypes. After gathering data from real patients, we were able to find the area under curves of over 80% in all comparisons. that electrical impedance dermography will be able to serve as a biomarker for non-melanoma skin cancers.
Introduction: Clinical diagnosis of basal cell (BCC) and squamous cell (SCC) carcinoma subtypes is challenging. Multiple subtypes of BCC and SCC can be difficult to distinguish clinically and ideally require different biopsy techniques for optimal histologic analysis and therapeutic decision-making [1]. Visual detection of BCC and SCC can be facilitated with the aid of dermoscopy [2] but determining prior to biopsy whether a lesion is superficial or more deeply invasive is usually not possible. There is a great clinical need to develop new technologies to augment visual skin examination to guide biopsy-decision-making and improve the management of lesions suspicious for BCC and SCC. To date, there is no bedside technique available that is low-cost, easily applied, quantitative, objective, and capable of overcoming these diagnostic hurdles. EID is a newer non-invasive, quantitative, and objective tool sensitive enough to detect alterations in the electrical properties of skin cancers. The overarching hypothesis of my proposal is that EID can be used to distinguish BCC subtypes and between SCC-in situ, invasive SCC, and inflamed keratosis that cannot be appreciated clinically. Clinical Diagnosis of BCC and SCC subtypes is challenging.
The “superficial” form of BCC is confined to the epidermis and can be effectively treated by non-surgical means. The “nodular” form of BCC consists of a collection of round tumor cells occupying the upper part of the dermis and can be treated by destruction or surgically depending on its size and location. “Micronodular” and “infiltrative” forms of BCC consist of smaller aggregates of tumor cells or angulated or stranded tumor cells, respectively, infiltrating the deeper dermis and usually requiring surgical treatment. Importantly, these invasive subtypes of BCC can present as papules or plaques that cannot reliably be distinguished clinically from nodular or the more superficial subtype of BCC. While the “shave” biopsy technique is more appropriate for superficial BCC, the “punch” biopsy is preferred for invasive subtypes. The superficial form of SCC can resemble BCC, and it is challenging clinically to distinguish this entity from invasive SCC; the former is best biopsied by shave technique and can be effectively treated non-surgically while the latter is best biopsied by punch technique to assess the depth of invasion and usually requires surgical excision [3]. These histologic changes cannot be reliably appreciated visually, and thus distinguishing subtypes of BCC and SCC presents a clinical conundrum. EID technology could contribute to overall clinical assessment by increasing confidence and diagnostic accuracy that will inform biopsy-decision making in patients with lesions suspicious of skin cancer.
Data Gathering:
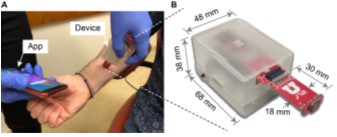
Using the innovative EID device as seen in Figure 1 and in collaboration with Dr. Douglas Grossman at the Huntsman Cancer Institute we gathered 17 BCC and 35 SCC lesions. Dr. Douglas Grossman is a Professor of Dermatology and Co-Leader of the Melanoma Center at the Huntsman Cancer Institute. He has seen patients for skin cancer screening and treatment for the past 25 years and has extensive experience in the use of non-invasive technologies.
Figure 1. URSKIN handheld electrical impedance dermography device (A) Plot use of the device in the clinic (B) The reduced dimensions allow the operator to hold the device with one hand while with the other hand controls the device with a smartphone.
Using Machine Learning to Classify Non-Melanoma Skin Cancers: Using R-Studio we trained a machine learning random forest algorithm, combining EID data with morphologic and histologic characteristics and tumor subtype, using the bootstrapping sampling method to reduce overfitting. We split our data into two parts for the learning algorithm: 80% assigned to the training data, and 20% assigned to the test set. We then performed a nested 10-fold cross-validation approach for prediction with random forest in two loops. An inner loop will be allocated to determine the individual training data estimates and their performance, whereas the outer loop will be used for checking the ability of these estimates in making predictions on the test set. Combining the performance of each test set from all the outer loops will provide the final evaluation output of the learning algorithm. Finally, we will create receiver operator characteristics curves to check our algorithm’s performance for each of the grouped datasets, with the area under the curve providing the probability of the given learning model’s ability to correctly classify.
Results:
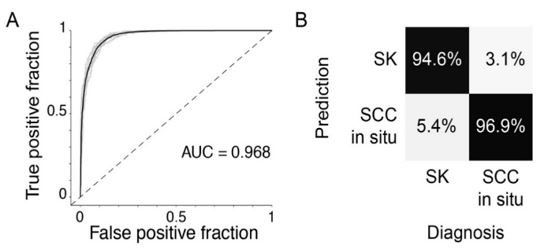
My results showed EID to be very effective and efficient at diagnosing BCC and SCC. In the BCC study, I obtained a specificity of 88%. Similarly, in the SCC study, I achieved an averaged area under the curve of 0.968, a sensitivity of 94.6%, and a specificity of 96.9% (Fig.1). In both cases, my results exceed the diagnostic accuracy of using dermoscopy, the clinical gold standard technology. I currently am the first author of the pilot manuscript under review at the Journal of Investigative Dermatology Innovations [4] describing the results of my work.
Additional results showed the comparisons of SCC in situ versus normal skin which had an area under the curve of 82.5%. Invasive SCC vs normal skin is 95.5% and invasive SCC vs SCC in situ is 99.9%.
Discussion and Conclusion:
These results, even with small datasets, have already shown promising results for bioimpedance as a biomarker for non-melanoma skin cancers. This will only improve with increased dataset size.
This research will advance medical and scientific fields by establishing the connection between histological and electrical properties of skin cancer lesions. My work will enable rapid and accurate diagnosis of skin cancers and their subtypes, which currently rely heavily on dermatologist expertise and open opportunities for this relationship to be used to diagnose both malignant and benign lesions non-invasively. EID could be applied to other dermatology applications beyond skin cancer detection including monitoring the effectiveness of treatment in individual patients and therapeutic research.
By establishing the histological-electrical relationship between healthy and diseased skin tissue, any person with a skin lesion can be diagnosed rapidly and accurately. My research will increase our understanding of electrical behavior in cancers non-invasively. My research will then be used to teach students translational applications of electrical engineering concepts to help people with medical conditions.
Future work for this research will include gathering more datasets for the SCC and BCC subtypes to make our machine-learning algorithms even more robust. Additional information will be gathered as well such as the specificity, sensitivity, and accuracy for each of the two-category measurements. Additionally, to improve its usage in the clinical environment the device and app that is being used would be updated to include the prediction resulting from the machine learning to give real-time predictions to the clinician.
Figure 2. (A) Receiver operator curve and (B) Confusion matrix of Machine Learning algorithm diagnosing SCC insitu and inflamed SK. Data from Wong et al., (4).
References:
[1] Cameron, M. C. et al. J Am Acad Dermatol 80, 321–339 (2019).
[2] Navarrete-Dechent, C. et al. JAMA Dermatol 156, 882 (2020).
[3] Motley, R. et al. British Journal of Dermatology 146, 18–25 (2002).
[4] Wong et al. Journal Investigative Dermatology Innovations, 2023.

