School of Medicine
51 Cervical Dorsal Root Ganglion Imaging for Studying Neuronal Nociceptive Circuit Changes in Migraine Models
Kara Andersen; Sarah Clair (Neurology); John Cheriyan; and K.C. Brennan (Neurology, University of Utah)
Faculty Mentor: K.C. Brennan (Neurology, University of Utah)
ABSTRACT
Migraine neuroscience is a field in its infancy, with migraine being a remarkably common yet poorly understood sensory circuit disorder. It is characterized by attacks of unilateral, throbbing craniofacial pain, with sensitivity to movement, visual, auditory, and other afferent inputs. Migraines can increase in frequency and intensity over time, transitioning from episodic to chronic migraines that resemble a never-ending migraine attack. The long-lasting, debilitating pain of migraine attacks is thought to follow a circuit of neuronal networks connecting the peripheral nervous system to the central nervous system as it ascends to the cerebral cortex for processing of painful stimuli. Using in vivo two-photon microscopy calcium fluorescence imaging with a novel surgical preparation to image fluorescence from genetically encoded calcium indicators in the C2 dorsal root ganglion (DRG), we establish an imaging paradigm of sensory neurons from the C2 DRG in response to greater occipital nerve and associated dermatome stimulation. We observe that in a nitroglycerin-induced chronic migraine state, a greater number of sensory neurons in the DRG respond when the greater occipital nerve dermatome is electrically stimulated. Additionally, this responder expansion is noted in sodium-nitroprusside-induced acute migraine models. Importantly, the understanding of nociceptive network activation in migraine will allow us to look at changes in the pain circuit that serve as markers for a migraine attack, observe how they occur, and see if they can be reversed at a neuron or circuit level to prevent the pain associated with migraine.
INTRODUCTION
Neuroscience became a distinct discipline of biological science in the late 1950s and early 1960s (Cowan et al., 2000). It has an interdisciplinary scope, combining expertise from physiology, anatomy, biology, cytology, computer science, and mathematical modeling in order to understand the nervous system. In addition to examining the normal development and activity of the nervous system, neuroscience studies diseases, disorders, and injuries that affect parts of the nervous system, how it develops, and how well it functions (Bear et al., 2016). Systems neuroscience—the study of the function of neural circuits in intact organisms—aims to understand how biological circuits in the brain generate behavior, thought, sensation, and action (Feldman & Scott, 2020). It is an integrative approach that allows deeper investigation into both healthy and abnormal nervous systems (Brennan & Pietrobon, 2018).
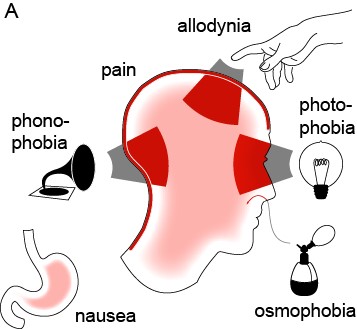
Migraine is an extremely common yet poorly understood nervous system disorder that primarily affects the sensory nervous system (Brennan & Pietrobon, 2018). According to the World Health Organization, “Migraine stands as the sixth most common cause of disability on the planet” (Goadsby et al., 2017). It affects 12% of the world’s population, and attacks are often incapacitating (Jensen & Stovner, 2008; Lipton et al., 2008). Migraine is characterized by attacks of unilateral, throbbing craniofacial pain, with sensitivity to movement, visual, auditory, and other afferent inputs (Headache Classification Committee of the International Headache Society, 2013). Most migraine attacks feature sensory amplifications: photophobia, phonophobia, osmophobia, and cutaneous allodynia—the perception of light, sound, smell, and normal touch as amplified or painful (Figure 1) (Burstein et al., 2015). Migraine is not simply a headache; the migraine attack is purely the most visible element on a larger continuum of disease.
Figure 1: The migraine attack involves changes in multiple sensory percepts (Brennan & Pietrobon, 2018).
Migraine attacks can increase in frequency over time. Headache experts divide states of progression into no migraine, episodic migraine, and chronic migraine. Chronic migraine is defined by the International Classification of Headache Disorders (ICHD-3) as 15 or more headache days per month. At least eight of those headaches must be migraine attacks, meaning they must be accompanied by one-sided, pulsing pain, increased pain with physical activity, light and/or sound sensitivity, and nausea (International Headache Society, 2013). Chronic migraine affects two percent of the general population, and studies suggest that three percent of people with episodic migraine will transition to chronic migraine each year (May & Schulte, 2016). Longlasting and/or repetitive pain over years leads to profound functional and structural changes in the brain networks (Brennan & Pietrobon, 2018). From an electrophysiological point of view, chronic migraine resembles a never-ending migraine attack (Coppola & Schoenen, 2012).
The incessant, debilitating pain because of migraine is the most prevalent symptom of all migraineurs. Acute onset and prolonged expression of pain are the leading symptoms that cause individuals to seek migraine treatment through the emergency department. 1.2 million emergency room visits are made in the United States each year seeking medical treatment for migraine (Dodson et al., 2018). In an emergency room situation, physicians prescribe narcotics as the primary treatment option for migraine visits more than 50 percent of the time, despite the fact that there is no specific indication for opiates in migraine treatment (Opioids and Migraine, 2018). In a 2019 study, 19 percent of people with migraine were currently using opioids prescribed by primary care providers specifically to treat migraine—up from the 16 percent reported in 2009 (Ashina et al., 2019). Using opioids to treat migraine has been found to lead to more frequent and severe headaches, as well as rebound headaches; to be a high risk factor in medication overuse headache; to trigger the transition from episodic migraine to chronic migraine; and, when consistently used, to easily become dependent upon and addicted to (Opioids and Migraine, 2018). Most crucially, doctors are treating the symptom of pain rather than the underlying disease of migraine, leading to no real solution.
Furthermore, while other acute medications that treat the potential inflammatory cause of migraine, such as triptans and non-steroidal anti-inflammatory drugs (NSAIDs), exist, a large population of people can’t take them, including the elderly, pregnant women, and anyone with a medical history of cardiovascular disease, diabetes, liver cirrhosis, kidney disease, or gastrointestinal bleeds (Opioids and Migraine, 2018). Some patients do not respond to triptans and other currently available medications. New studies have shown exploration into 5-HT1F receptor blockers and calcitonin gene-related peptide (CGRP) receptor antagonist medication options to treat migraine as they have less cardiovascular risk for patients, but many uncertainties still exist (de Prado & Russo, 2006; Neeb et al., 2010). This means there’s a bleak number of viable treatment options to acutely relieve pain and treat migraine that work for everyone, and people deserve to have treatment plans that are effective.
While new data is emerging about the pathophysiology of this debilitating disease (Olesen et al., 2009), little is known about the nociceptive circuit associated with a migraine attack, and in order to truly treat migraine and relieve its most prevailing symptom, the pain circuit must be understood at a deeper level. As a craniofacial pain disorder, migraine is classically considered “trigeminal” in nature, meaning focused within the trigeminal nerve (Brennan & Pietrobon, 2018; Edvinsson et al., 2020). Yet half of the head is covered by the greater and lesser occipital nerves. An emerging consensus is that all sensory nerves covering the head, including the trigeminal and upper cervical networks, can serve as pain substrates and that their effects synergize (Brennan & Pietrobon, 2018). The head pain associated with a migraine attack, including the frontal, temporal, parietal, occipital, and high cervical regions, is thought to be the consequence of activation of the trigeminovascular system and the dorsal root ganglia of cervical roots C1–C3 (Brennan & Pietrobon, 2018; Goadsby et al., 2017). The greater occipital nerve (GON) is the medial branch of the dorsal ramus of the second spinal nerve (C2) (Nielsen, 2020), and clinical GON blocks have shown to have a beneficial effect on chronic migraine preventative pain treatment (Chowdhury et al., 2021). From an experimental surgical standpoint, the GON circuit offers a critical advantage: it is all accessible—nerve and associated cutaneous territory, dorsal root ganglion, and dorsal horn (Brennan & Pietrobon, 2018). This allows for less invasive surgeries, reduced pain, and decreased potential complications when exploring the migraine pain circuit.
Nociceptive circuitry is the arrangement and functional interconnections of neurons that are responsible for conveying pain information (Todd, 2013). Neurons are differentiated and specialized, and they can be categorized into afferent and efferent neurons based on the function they provide. Afferent or sensory neurons are activated when they receive sensory input from the environment, such as touch, pressure, temperature, light, smell, taste, or pain. They transmit information about changes in homeostasis to the brain. Efferent or motor neurons are employed when the body wants to initiate a response to a stimulus. They send messages from the central nervous system to effector organs that can then carry out actions that restore equilibrium (Bear et al., 2016; Tortora & Nielsen, 2017). A neuron can receive contacts from up to 10,000 presynaptic neurons, and, in turn, a neuron can communicate with up to 10,000 postsynaptic neurons in order to pass information along (Byrne, 1997). The number of possible neuron combinations is what gives rise to complex neuronal circuits and networks that are currently under investigation.
Pain perception circuits begin with free nerve endings, which are branches of the primary neuron. The multitude of different receptors, including nociceptors (pain receptors), convey information that converges onto neuronal cell bodies located in the dorsal root ganglion for stimulus from the body and the trigeminal ganglia for stimulus from the face (Handler & Ginty, 2021; Kendroud et al., 2021).
Though new genetic markers reveal a multitude of different neuronal types (Handler & Ginty, 2021), there are two major types of nociceptive nerve fibers: A-delta fibers and C-fibers. A-delta fibers are lightly myelinated and have small receptive fields, which allow them to alert the body to the presence of pain. Due to the higher degree of myelination compared to C-fibers, these fibers are responsible for the initial perception of pain. C-fibers, on the other hand, are unmyelinated and have large receptive fields, which allow them to relay pain intensity (Arcilla & Tadi, 2022; Bear et al., 2016; Kendroud et al., 2021).
Nociceptive stimuli activate transient receptor potential (TRP) channels located on nerve endings, which cause first-order neurons to depolarize and fire action potentials. First-order neurons are found in the dorsal root and trigeminal ganglions, which are both considered parts of the peripheral nervous system (Kendroud et al., 2021; Nielsen, 2020; Yam et al., 2018). When receiving pain sensory information from the body, second- order neurons decussate at connections with the central nervous system and then ascend via the lateral spinothalamic tract (Bear et al., 2016; Kendroud et al., 2021; Nielsen, 2020). Third-order neurons are located in the ventral posterolateral nucleus (VPL) and the ventral posterior inferior nucleus (VPI). From the thalamus, nociceptive information projects to the primary somatosensory cortex for further processing and pain perception (Kendroud et al., 2021; Nielsen, 2020). This is the proposed ascending circuit pathway that researchers continue to make inquiries about.
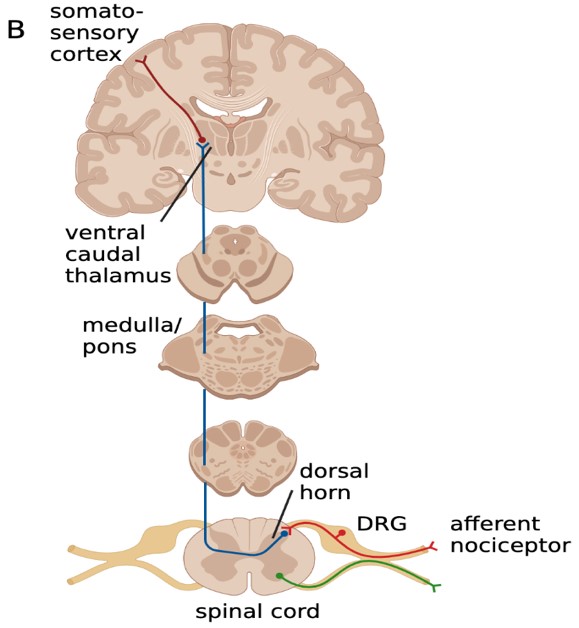
This project focuses on the neural circuits involved in the manifestation of the migraine phenotype, specifically the ascending circuit pathway (Figure 2) that may involve the cervical dorsal root ganglion (DRG) and central circuits. We aim to understand the nociceptive circuit associated with a migraine attack and the changes that occur in the pain circuit when it becomes a chronic migraine state.
Figure 2: The ascending circuit pathway may be involved in the manifestation of the migraine pain phenotype.
METHODS
All animal procedures are performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah as consistent with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Part 1: Mice Strain
Mice are often used as animal models in neurology because their genetic, biological, and behavioral characteristics closely resemble those of humans in relation to the basic features of pain (Hodge et al., 2019). While non-animal methods of study have made progress in some fields of biomedical research, their use in neuroscience remains extremely limited due to the complex and interconnected structure of the brain. In most cases, a living and behaving organism remains the only viable model to study the brain in action (Jones, 2021).
To selectively study the dorsal root ganglion (DRG), we are using Pirt-1 Cre x Ai95 transgenic mouse lines (Kim et al., 2016). Pirt is a modulator of several transient receptor potential (TRP) channels. Cre recombinase is expressed specifically in Pirt positive neurons, meaning all primary sensory neurons in the DRG and not in any central nervous system neurons (Kim et al., 2008). This allows for direct genetic manipulation and study of DRG neurons. The Ai95 mice supply a genetically encoded calcium indicator, GCaMP6f, that has been widely used for imaging calcium (Ca2+) transients in neuronal somata, dendrites, and synapses of the peripheral nervous system. In theory, this indicator is specific to the peripheral nervous system, which is essential to studying dysfunction of the sympathetic nervous system with regard to migraine (Anderson et al., 2018).
We use both male and female mice that are between 8 and 24 weeks old. This ensures that the mice are mature adults but have not suffered any negative age-related changes, including loss of locomotor activity, sensory and motor function changes, anxiety-like and depression-related behaviors, and declines in learning and memory.
Part 2: Chronic NTG Migraine Model
Nitroglycerin (NTG) is a vasodilator drug commonly used in the treatment of chest pain and high blood pressure. Since its primary course of action is to dilate blood vessels, an extremely common side effect of this medication is a nitroglycerin-induced headache, though the actual mechanism of how these are related is still unknown (Bektas & Soyuncu, 2010; Sureda-Gibert et al., 2022). In migraineurs, NTG induces a severe delayed headache, resembling a spontaneous migraine attack. Due to this known human migraine trigger, scientists developed a model of chronic migraineassociated pain. Chronic, intermittent administration of NTG to mice has been shown to result in hyperalgesia—an abnormally heightened sensitivity to pain (Moye & Pradhan, 2017).
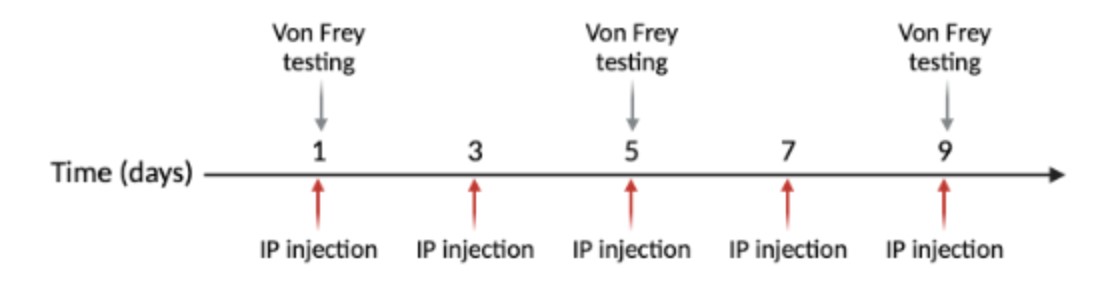
For purposes of the experiment, half the animals received vehicle (control) injections and half received NTG. Systemic intraperitoneal injections occur every other day for nine days, with five injections total (Figure 3). The NTG is dosed at 10 mg/kg and administered at 10 mL/kg (Moye & Pradhan, 2017). NTG (in 30% alcohol, 30% propylene glycol, American Reagent, NY, USA) is diluted each time in 0.9% saline prior to administration. Vehicle is 30% alcohol, 30% propylene glycol, in saline.
Figure 3: Injections are done on days 1, 3, 5, 7, and 9. Von Frey behavioral testing is performed on days 1, 5, and 9.
Part 3: Behavior Testing
Acclimation of the animals to the behavior testing room and testing conditions is crucial to reducing confounding variables. This room should be quiet, with low-light conditions, and minimal disruptions during testing to minimize animal stress and variability.
Since pain experience is subjective by nature and cannot be measured directly, pain in animals is inferred based on pain-like behaviors such as withdrawal. Migraine attacks are usually accompanied by sensory amplifications and cutaneous allodynia (a type of pain where touch that usually isn’t painful causes severe pain) (Burstein et al., 2015). Manual Von Frey testing is a way to test allodynia in mice via hind paw withdrawal to a pain stimulus. It is completed using monofilaments of differing forces, ranging from 0.008 to 2 grams. Each stimulus is applied perpendicularly to the hind paw. If the rodent withdraws, licks, or shakes the paw, it is considered to have had a positive response to pain. If the animal does not give a response, the next higher gram force filament is used to repeat the procedure and check for a response. If the animal does respond, the procedure is repeated with the next lower gram force filament (Deuis et al., 2017). A basal threshold (baseline) should be completed one hour prior to injections and repeated one-hour post-treatment.
This testing procedure is performed on days one, five, and nine of the experiment to avoid associative learning (Figure 3) (Moye & Pradhan, 2017).
Part 4: Surgery
For less than a decade, surgeries have been performed on mice to view and study the lumbar DRG (Caylor et al., 2019; Chen et al., 2019; Chen et al., 2022). This current study applies similar surgical techniques to the cervical vertebrae to create a novel procedure that allows in vivo imaging of the C2 DRG (Figure 4).
Figure 4: In vivo two-photon microscopy fluorescence imaging of C2 DRG with greater occipital nerve (GON) stimulation.
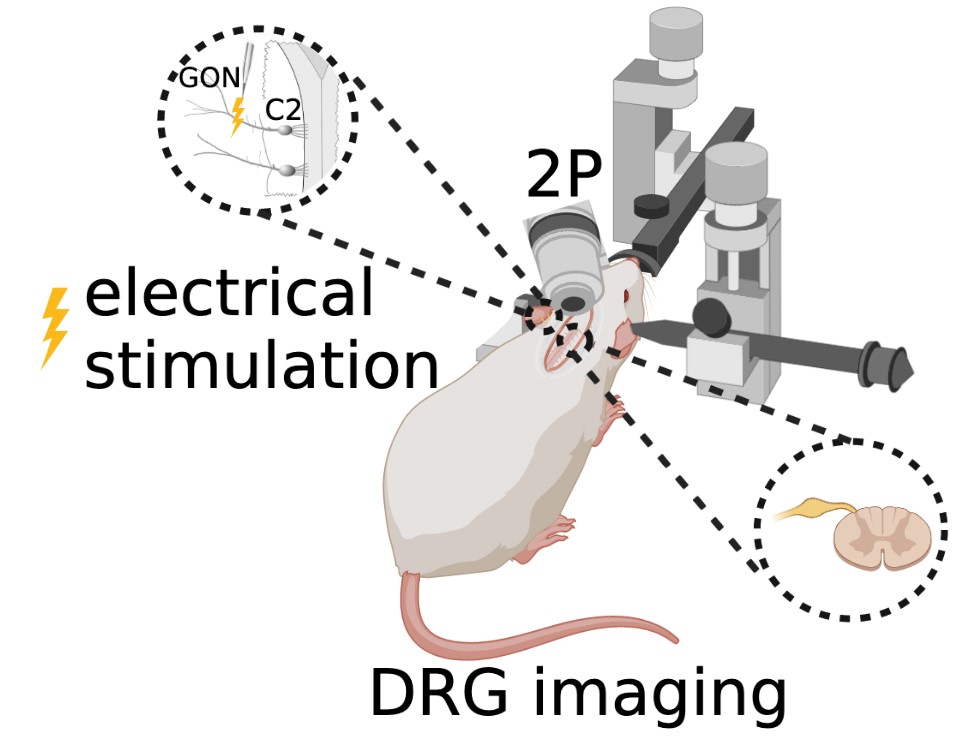
The animal is maintained under deep anesthesia with 1.5–2.5% isoflurane. Anesthesia is verified by the absence of the toe pinch reflex. Ocry-gel is applied to both eyes to maintain eye moisture and prevent corneal abrasions. After shaving the fur at the surgical site and sterilizing the skin with 70% ethanol and 10% povidone-iodine, a small incision is made in the dorsal skin from levels C1– C4 of the spine, and the skin is held back with sutures. Under a stereomicroscope, muscles and Figure 4: In vivo two-photon microscopy fluorescence imaging of C2 DRG with greater occipital nerve (GON) stimulation. ligaments attached to the lateral aspects of the first three vertebrae are detached using surgical scissors. A laminectomy of the C2 vertebra is performed to surgically remove both spinous and transverse processes of the vertebral bone in order to expose the C2 region of the spinal cord and DRG. Once dissected and cleaned away from any remaining fat, meninges, and muscle, the C2 DRG should be clearly visible. A titanium head bar is mounted on the skull in a configuration that allows for stability and clarity of the DRG field of view while imaging.
In vivo two-photon microscopy fluorescence imaging of the genetically encoded calcium indicator GCaMP6f is completed using an immersion objective in an anesthetized state coupled with occipital nerve stimulation.
Part 5: Acute NTG Migraine Model
Retroorbital injections of either NTG (10 mg/kg) or vehicle (6%v/v ethanol, 6%v/v propylene glycol in saline) are given after the mouse is transferred from the surgical station to the imaging platform. A 30-gauge needle connected to a cannula is inserted into the retrobulbar space and positioned to remain in place throughout the course of the imaging. After recording the pretreatment stimulation responses, approximately 100μL of NTG or vehicle solution is injected through the cannula. Post-treatment responses are collected 30 minutes after the injection.
Part 6: Acute SNP Migraine Model
Sodium nitroprusside (SNP), like NTG, is a nitric oxide (NO) donor that has similar effects on sensory behavior (Divakaran & Loscalzo, 2017). Unlike NTG, SNP is water soluble, thus avoiding potential spurious effects from solvents used as vehicle. After the mouse is transferred from the surgical station to the imaging platform and pre-treatment stimulation responses are recorded, a 30-gauge needle connected to a cannula is inserted into the retrobulbar space and positioned to remain in place throughout the rest of the course of imaging. SNP (2 mg/kg) is dissolved in PBS, and 100μL of SNP solution or PBS vehicle is injected into the retroorbital space. Post-treatment responses are collected at 30- and 60-minutes post-injection. (See experiment scheme below.)
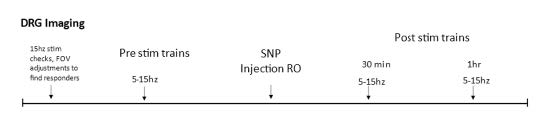
Part 7: Electrical Stimulation
Electrical stimulation is performed with monopolar needle electrodes. The singular electrode is placed against the skin, near the fold between the ear and the neck, to stimulate the greater occipital nerve branching in the C2 dermatome. All pulse parameter paradigms are described below in Figure 5.
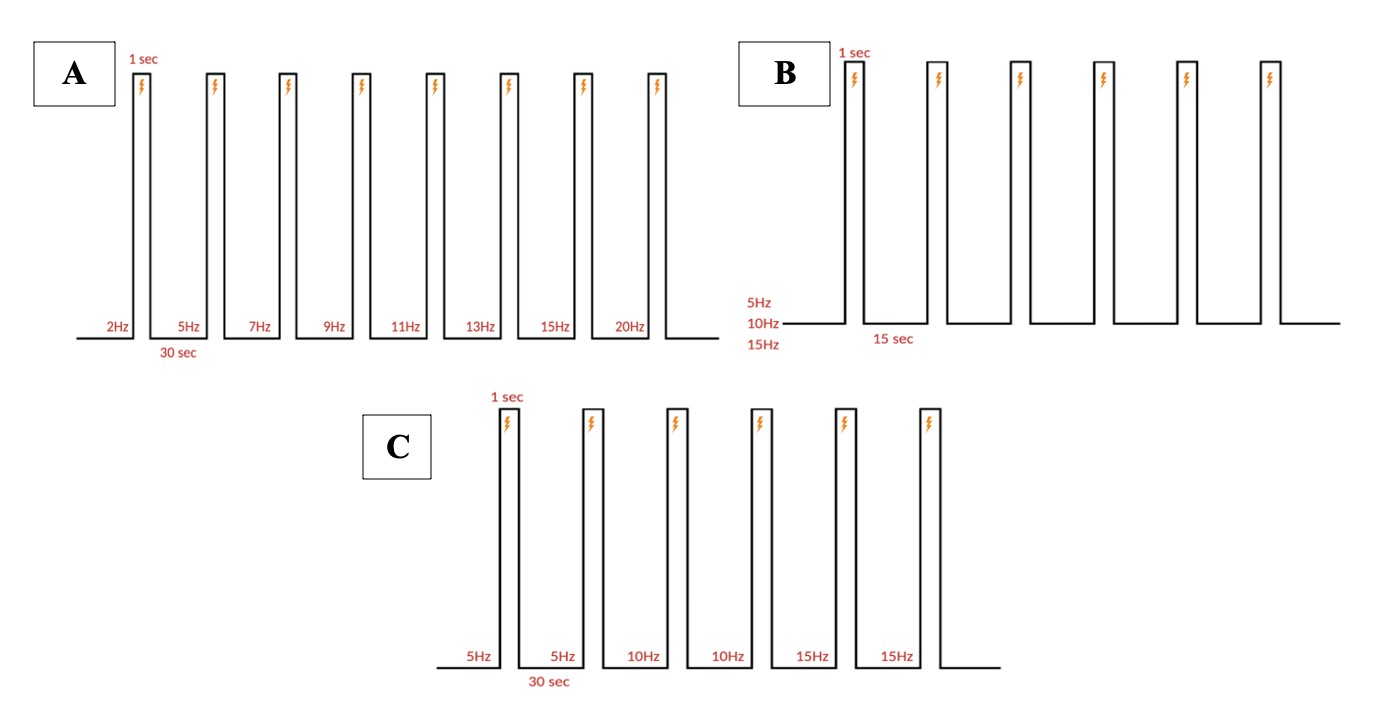
Figure 5: Stimulation paradigm. A. Chronic and acute NTG experiment parameters. 1 ms pulse duration of 0.3 mA at 5, 10, or 15 Hz for a 1 second burst width is repeated six times (at a single frequency) every 15 seconds for one recording. B. Acute SNP experiment parameters. 10 ms pulse duration of 0.3 mA at 5, 10, and 15 Hz for a 1 second burst width, two times at each ascending frequency, every 30 seconds for one recording. C. Acute NTG experiment parameters. 10 ms pulse duration of 0.3 mA at 2, 5, 7, 8, 11, 13, 15, and 20 Hz for a 1 second burst width, once at each ascending frequency, every 30 seconds for one recording.
Part 8: Data Analysis
Images are recorded from pain-encoding neurons in the DRG, where regions of interest (ROI) of responding cells are determined visually and programmatically (Figure 6A). A critical part of action potentials is the influx of intracellular calcium (Park & Luo, 2010). Calcium entering the neuronal cell throughout these channels is associated with transduction, transmission, processing, and modulation of pain signals (Castro-Junior et al., 2018). The genetically encoded calcium indicator, GCaMP6f, enables recording of the calcium dynamics in the DRG cells in response to the electrical stimulus (Figure 6B). ROI are then converted to raw fluorescence traces in ImageJ using inbuilt and custom-built macros. Raw image stacks are converted to changes in fluorescence Figure 5: Stimulation paradigm. A. Chronic and acute NTG experiment parameters. 1 ms pulse duration of 0.3 mA at 5, 10, or 15 Hz for a 1 second burst width is repeated six times (at a single frequency) every 15 seconds for one recording. B. Acute SNP experiment parameters. 10 ms pulse duration of 0.3 mA at 5, 10, and 15 Hz for a 1 second burst width, two times at each ascending frequency, every 30 seconds for one recording. C. Acute NTG experiment parameters. 10 ms pulse duration of 0.3 mA at 2, 5, 7, 8, 11, 13, 15, and 20 Hz for a 1 second burst width, once at each ascending frequency, every 30 seconds for one recording. A C B (ΔF = Ft – F0), where Ft is the fluorescence intensity of a given frame and F0 is the average fluorescence of the first 3-5 seconds. Traces are normalized as ΔF/F0 and analyzed using Matlab.
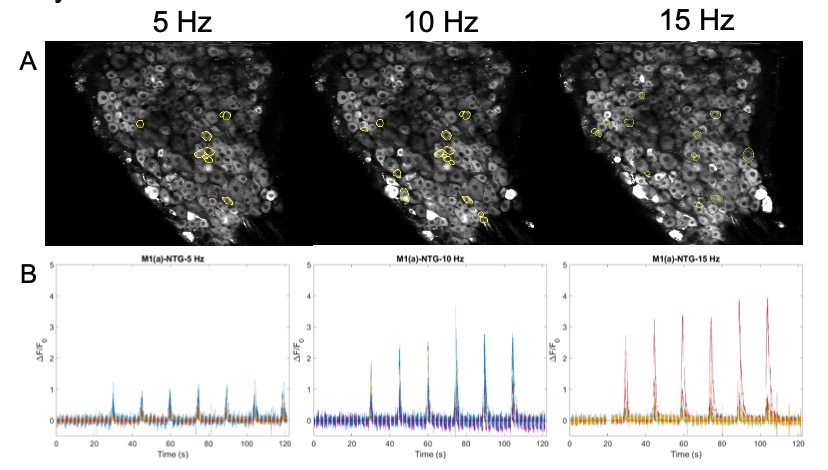
Figure 6: A. Images of DRG with circled ROI. B. Calcium fluorescence traces of responding neurons according to 5, 10, 15 Hz stimulation protocol.
Increased baseline sensitivity for tactile behavior in the chronic NTG model
NTG at 10 mg/kg, or vehicle, every other day for five times over a total period of nine days was injected intraperitoneally in the chronic model. Baseline measurements of mechanical sensitivity were carried out before NTG administration on days 1, 5, and 9 (Figure 7A). Days five and nine showed a significant reduction in paw withdrawal threshold, indicating the persistent hypersensitization induced by chronic NTG dosage (Two-way ANOVA: NTG days 5 and 9, p=0.04 and p=0.03 respectively). However, one- hour post-treatment responses showed sensitization in both the vehicle and NTG groups (p=0.06, Two-way ANOVA) (Figure 7B).
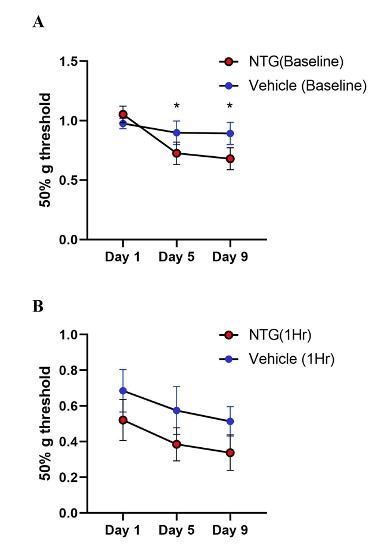
Figure 7: Tactile behavior in chronic NTG treatment model on Ai95/Pirt-1 mice. A. Baseline paw withdrawal thresholds before NTG treatment (10 mg/kg) on days 1, 5, and 9. (N=7 mice in NTG group, N=9 mice in vehicle control group). The baseline values on Day 5 and Day 9 were significantly different than Day 1 in the NTG group and not in vehicle group (p=0.04 day 5, p=0.03 day 9, Two-way ANOVA). B. Paw withdrawal thresholds in chronic NTG and vehicle groups at 1-hr post- treatment. NTG withdrawal thresholds compared to vehicle group showed no significant difference (p=0.06, Two- way ANOVA).
Increased tactile sensitivity in acute SNP treated mice
Our behavior assays with acute SNP treatment showed a clear distinction in tactile sensitivity between the compound treated group and vehicle control (Figure 8). To optimize our acute SNP dosage for our imaging prep, we tested two doses of SNP: 2 mg/kg (p=0.02, Two-way ANOVA) (Figure 8A) and 2.5 mg/kg (p=0.13, Two-way ANOVA) (Figure 8C). Our goal was to identify the ideal dose that induces nociceptive effects without confounding the compound’s vasodilatory effects. Additionally, SNP releases cyanide during metabolism, and cyanide toxicity is a potential, though rare, complication (Rindone & Sloane, 1992). Since both doses induced tactile sensitivity as recorded by the von Frey test, 2 mg/kg was chosen for our acute experiments involving in vivo imaging of C2 DRG neurons to reduce risk while still giving a behavioral response.
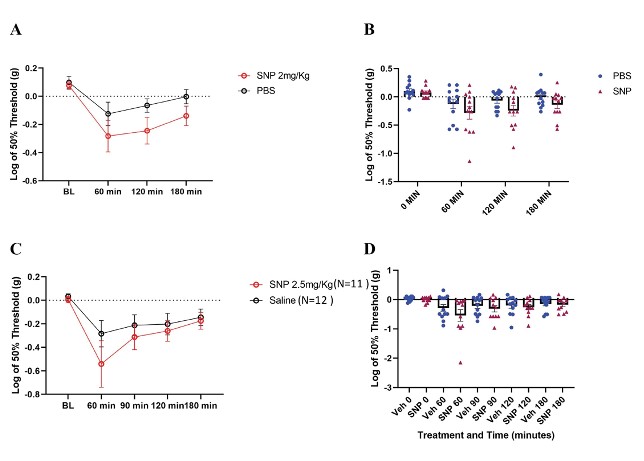
Figure 8: Tactile behavior assay outcomes in C57BL/6 mice treated with SNP. A/B. Paw withdrawal thresholds from N=12 mice each in SNP (2 mg/kg dose) and vehicle. (p=0.02, Two-way ANOVA). C/D. Paw withdrawal thresholds in SNP treated (2.5 mg/kg dose) and vehicle treated mice (SNP N=11 mice; vehicle N=12 mice) (p=0.13, Two-way ANOVA).
C2 DRG responses in the chronic and acute NTG models show subtle differences Chronic NTG model – In vivo GCaMP calcium fluorescence imaging for Ai95/Pirt1-Cre mice C2 DRG from chronic NTG and vehicle groups were completed within the first five days following the last intraperitoneal injection day (day 9). This ensured that the experiments were performed within the chronic NTG-induced sensitization period as reported by other groups (Moye & Pradhan, 2017). Calcium transient responses were recorded from DRG cells according to the stimulation paradigms.
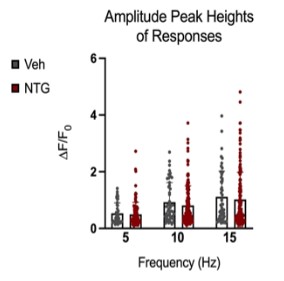
Figure 9: Comparison of peak amplitudes of calcium responses in C2 DRG of chronic NTG/Vehicle treated mice. Responses to six repeat stimulation trains at 5Hz, 10Hz, and 15Hz frequencies of 0.3mA current pulses. The ROIs from all the responding cell populations are plotted (NTG group, N=163 cells, n=14 mice; Vehicle group, N=54 cells, n=10 mice). ΔF/F0=(F-F0)/F0
Peak amplitudes of calcium fluorescence in both NTG and vehicle groups were analyzed (Figure 9). No major differences were observed in response peak amplitudes between the chronic NTG group and the vehicle group. Ca2+ responses to C2 DRG stimulation in the chronic migraine model mice versus control vehicle administered mice were also evaluated for expansion of responding cells. When in a nitroglycerine-induced chronic migraine state, a greater percentage of sensory neurons in the DRG respond when the greater occipital nerve dermatome is electrically stimulated (p<0.05, unequal variance t-test) (Figure 10).
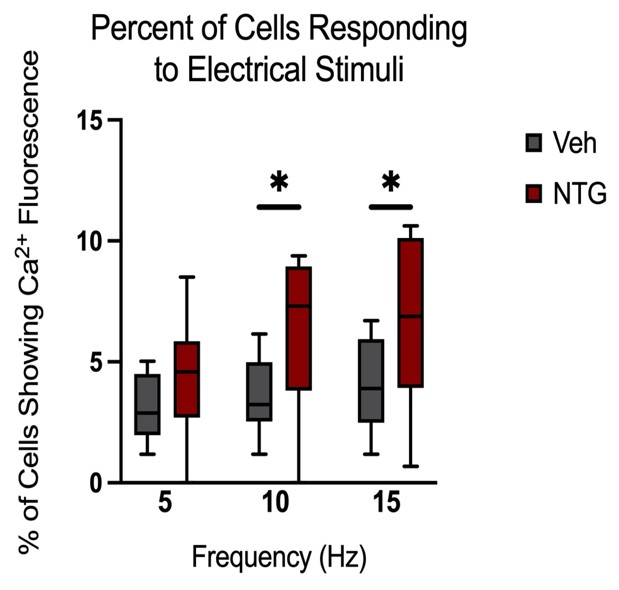
Figure 10: Comparison of the percentage of cells showing fluorescence (Vehicle n=10 mice, NTG n=14 mice). Responses to six repeat stimulation trains at 5Hz, 10Hz, and 15Hz frequencies of 0.3mA current pulses (p<0.05, unequal variance t-test).
Acute NTG model – A single dose of NTG (10 mg/kg) or vehicle was administered retro- orbitally during an in vivo imaging session. Responses to stimulation paradigms were collected before and after NTG or vehicle injection. The data was analyzed as an average of all response traces between NTG and vehicle groups. We found subtle differences between the NTG pre-treatment response and the NTG post-treatment response (p=0.06, Two-way ANOVA) (Figure 11C). The vehicle group did not show any differences between pre-treatment amplitudes and post-treatment values (Figure 11D).
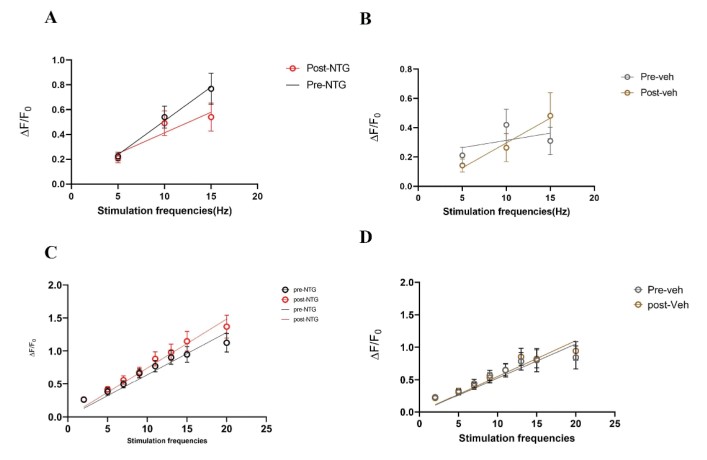
Figure 11: Comparison of peak amplitudes of calcium responses in C2 DRG of acute NTG/Vehicle treated mice to two stimulation paradigms. A. Responses to six repeat stimulation trains at 5Hz, 10Hz, and 15Hz frequencies of 0.3mA current pulses in the NTG treated mice. The mean±SEM of all ROIs from all the responding cell populations are plotted (NTG group, N=5 mice). B. Responses to six repeat stimulation trains in the vehicle group (Vehicle group, N=5 mice). C. Responses to range of frequencies from low 2Hz to high 20Hz with 0.3mA current pulses in the NTG group (p=0.06, Two-way ANOVA). D. Responses to 2-20Hz range of frequencies in the acute vehicle group.
Expansion of responders post-SNP treatment
Our behavior assays with acute SNP treatment showed a clear distinction in tactile sensitivity between the compound treated group and the vehicle control group (PBS/saline) (Figure 8). A dose of 2 mg/kg SNP was delivered retro-orbitally while concurrently imaging the C2 DRG. Responses to stimulation paradigms were collected before and after SNP or vehicle injection, and the data was analyzed for response peak amplitudes and the expansion of responding cells. We did not see a significant change in the response amplitude of responding cells to the frequency ranges of 5–15 Hz. Interestingly, our preliminary observations show an increase in the number of new responding cells in the SNP treated group (Figures 12–14). We compared the response traces from the same cell ROIs within an FOV before retroorbital administration of SNP and after. The new responders had low amplitude responses and were mostly identifiable at higher frequencies of 10 and 15 Hz (N=6 mice for SNP group, N=4 for vehicle group).
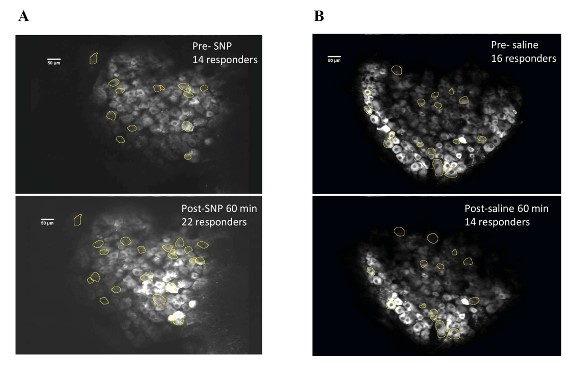
Figure 12: DRG cell responders before and after SNP/Vehicle treatment. A. Upper panel shows a representative FOV of responders before SNP and lower panel shows same FOV with responders post retroorbital administration of SNP. B. Upper panel shows an FOV before vehicle and lower panel shows same FOV after vehicle PBS administration in the retroorbital space.
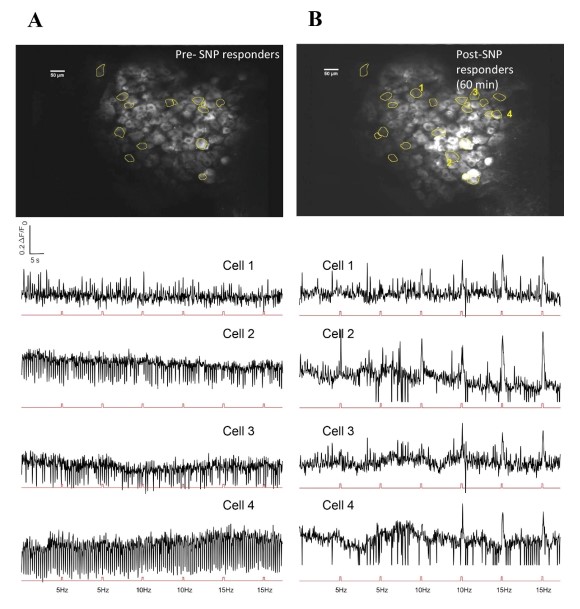
Figure 13: Representative image of DRG neurons and response traces to electrical stimuli before and after SNP. A. Upper panel shows FOV with responder cells before SNP. Lower panel has selected cell traces corresponding to those marked in B (Cells 1-4 in B). Stimulation pulses are represented in the red trace below each cell trace. B. Upper panel has same FOV after SNP administration, with representative new responders marked. Lower panel has corresponding responder peaks aligned to stimulation pulse sets shown below each cell trace.
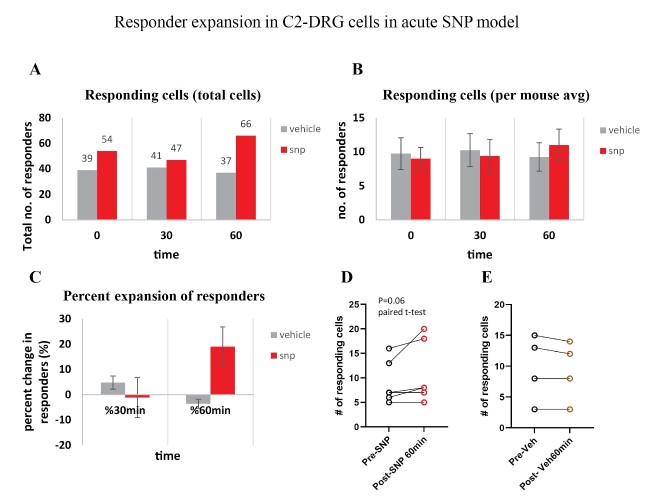
Figure 14: Responder expansion in cervical DRG in SNP treated mice. A. Total responding cells before SNP/Vehicle treatment (0 min), at 30-min and 60-min post-treatment. N=6 mice for SNP group, N=4 for vehicle group. B. Average of responding cells per mouse in each SNP or vehicle group. C. Percentage of responder increase at 30 min and 60 min compared to before treatment. D. Comparison of the number of responder cells from each mouse plotted before and after treatment with SNP (p=0.06, paired t-test). E. Comparison of the number of responder cells from each mouse plotted before and after treatment with vehicle.
DISCUSSION
Migraine is a disease that primarily affects the sensory nervous system. Along with other symptoms, migraine is characterized by attacks of unilateral, throbbing craniofacial pain. This pain is thought to be the consequence of activation of the trigeminovascular system and the dorsal root ganglia of cervical roots C1–C3 (Brennan & Pietrobon, 2018). We set out to investigate the ascending circuit pathway that may be involved in the manifestation of the migraine pain phenotype. We started our study of the nociceptive circuit at the C2 DRG due to surgical accessibility and compared neuronal activity changes in both chronic and acute headache models.
NTG is an effective migraine trigger in humans (Karsan et al., 2017). It has also been established as a model of chronic migraine-associated pain in mice (Moye & Pradhan, 2017). Indeed, we observe progressive and sustained mechanical allodynia at baseline following a chronic NTG treatment model, indicating persistent hypersensitization. Similar tactile allodynia results are seen with an acute SNP-induced headache model (Avona et al., 2020; Wattiez et al., 2021). This behavioral assay is consistent with the cutaneous allodynia reported in many migraine patients (Burstein et al., 2015).
Our experience with NTG encountered confounding variables. We see persistent tactile sensitization behavior in mice that underwent chronic dosing of NTG; however, one- hour posttreatment responses show sensitization in both vehicle and NTG groups. We think that the vehicle composition for NTG is a potential confound. The vehicle composition for NTG contains 30% propylene glycol and 30% alcohol. Propylene glycol has been shown to activate transient receptor potential (TRP) channels (Niedermirtl et al., 2018). Activation of TRP channels promotes excitation of nociceptive afferent fibers that potentially lead to pain (Dussor et al., 2014). Alcohol can activate TRPV1 channels that provide a sensation of heat and pain (Trevisani et al., 2002). Hence, the nociceptive effects induced by propylene glycol and alcohol could be a potential reason for the noisy behavior in both NTG and vehicle treated mice after one hour. The time course of our acute experiments also falls within this time span of activation and excitation, which may have negatively affected the outcomes.
We observe behavioral hypersensitivity to pain in the chronic NTG model. When we examine how C2 DRG neurons respond to the NTG challenge (hypersensitivity), we observe increases in the number of neurons responding to stimulation, indicating a change in the sensitivity of neurons at the C2 level. While observations in the chronic model show an expansion of responders in correlation with administration of NTG, this leads to the need for an acute headache model. The acute model allows us to investigate the effect of drug administration after establishing a baseline on the same animal, minimizing confounding variables. Similar patterns are seen in the preliminary data of acute models with the expansion of responders post administration of SNP.
Even though we did not see significant changes in the response amplitude of responding cells after acute administration of SNP, our initial observations show an increase in the number of new responding cells in the SNP treated group. This increased sensory neuron activation could be correlated with pain behaviors (Zheng et al., 2022), as more sensory neuron responses could mean potential amplification of signals in the ascending circuitry, meaning increased sensitivity. This phenotype appears to be similar to what has been seen in other pain models. Studies found that peripheral neuropathic pain models and complete Freund Adjuvant (CFA)-induced inflammatory pain models show an increase in activated neurons in L4 DRG surgical preparations post nerve injury or administration of CFA (Kim et al., 2016). These studies involved longer, chronic exposure to nerve injury or inflammation than our acute model, which could account for the lower extent of the responder expansion in our observations. While these results have been shown in other pain models, to our best knowledge, we offer the first results in a cervical DRG.
The low magnitude of calcium fluorescence changes we observe in the chronic NTG or acute NTG and SNP treatment models may align with the view that sensory neurons act as faithful relay centers, resisting major changes in activation. The all-or- nothing law is an important principle: no matter how weak the noxious stimuli are, once it reaches a certain threshold, the sensory neuron action potential will fire at full strength (Adrian, 1914). However, the lack of major increase in calcium fluorescence trace amplitude does not imply an absence of sensory changes. Furthermore, we do see subtle changes in the number of responding cells, which could have amplified effects at the dorsal horn or brainstem level that we have proposed to investigate. In vivo DRG imaging is the starting point for studying the role of primary sensory neurons in different somatosensations, including pain. It may thus be even more important to identify the origin of these changes in DRG because of targeting, which could lead to more specific therapies that prevent downstream amplification of sensory processing.
We ultimately aim to dissect the role of specific cell types within the C2 DRG involved in headache phenotypes of varying origins. Our experiments are currently limited in assessing the sensory neuronal classes that may be involved in the migraine pain mechanisms. For example, our electrical stimulation paradigm targeting the greater occipital nerve (GON) trunk region could activate multiple neuronal types relaying a mix of sensory modalities—not just pain. Additional experiments involving defined sensory modalities to probe specific cell types involved and their respective receptive fields would allow us to delineate peripheral circuitry critically involved in the migraine pain phenotype.
This project lays the groundwork for many subsequent questions. Future directions include performing C2 DRG afferent cell subtype-specific studies, identifying migraine relevant alterations in C2 DRG in acute models of migraine, studying migraine relevant alterations in DRG to CNS synapses, and investigating sex differences in migraine responses at a cellular level. Importantly, the understanding of nociceptive network activation in migraine will allow us to look at changes in the pain circuit that serve as markers for a migraine attack, observe how they occur, and see if they can be reversed at a neuron or circuit level to prevent the pain associated with migraine.
REFERENCES
Adrian, E. D. (1914). The all-or-none principle in nerve. J Physiol, 47(6), 460-474. https://doi.org/10.1113/jphysiol.1914.sp001637
Anderson, M., Zheng, Q., & Dong, X. (2018). Investigation of Pain Mechanisms by Calcium Imaging Approaches. Neurosci Bull, 34(1), 194-199. https://doi.org/10.1007/s12264-017-0139-9
Arcilla, C. K., & Tadi, P. (2022). Neuroanatomy, Unmyelinated Nerve Fibers. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK554461/
Ashina, S., Foster, S. A., Nicholson, R. A., Araujo, A. B., Reed, M. L., Shapiro, R. E., Buse, D. C., Jaffe, D. H., Cambron-Mellott, M., Li, V. W., Zagar, A., Pearlman, E. M., & Lipton, R. B. (2019). Opioid Use Among People with Migraine: Results of the OVERCOME Study. Headache, 59(S1), 208. https://doi.org/https://doi.org/10.1111/head.13549
Avona, A., Mason, B. N., Lackovic, J., Wajahat, N., Motina, M., Quigley, L., Burgos- Vega, C., Moldovan Loomis, C., Garcia-Martinez, L. F., Akopian, A. N., Price, T. J., & Dussor, G. (2020). Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptidedependent hyperalgesic priming to a migraine trigger. Pain, 161(11), 2539-2550. https://doi.org/10.1097/j.pain.0000000000001953
Bear, M. F., Connors, B. W., & Paradiso, M. A. (2016). Neuroscience: Exploring the Brain (Fourth Edition ed.). Wolters Kluwer. Bektas, F., & Soyuncu, S. (2010). Nitroglycerin-induced migraine type headache: bilaterally visible temporal arteries. Int J Emerg Med, 3(4), 463-464. https://doi.org/10.1007/s12245-010- 0248-y
Brennan, K. C., & Pietrobon, D. (2018). A Systems Neuroscience Approach to Migraine. Neuron, 97(5), 1004-1021. https://doi.org/10.1016/j.neuron.2018.01.029
Burstein, R., Noseda, R., & Borsook, D. (2015). Migraine: multiple processes, complex pathophysiology. J Neurosci, 35(17), 6619-6629. https://doi.org/10.1523/JNEUROSCI.0373- 15.2015
Byrne, J. H. (1997). Introduction to Neurons and Neuronal Networks Castro-Junior, C., Ferreira, L., Delgado, M., Silva, J., & Santos, D. (2018). Role of Calcium Permeable Channels in Pain Processing. Intechopen. https://doi.org/10.5772/intechopen.77996
Caylor, J., Reddy, R., Yin, S., Cui, C., Huang, M., Huang, C., Ramesh, R., Baker, D. G., Simmons, A., Souza, D., Narouze, S., Vallejo, R., & Lerman, I. (2019). Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med, 5. https://doi.org/10.1186/s42234-019-0023-1
Chen, C., Zhang, J., Sun, L., Zhang, Y., Gan, W. B., Tang, P., & Yang, G. (2019). Long- term imaging of dorsal root ganglia in awake behaving mice. Nat Commun, 10(1), 3087. https://doi.org/10.1038/s41467-019-11158-0
Chen, L., Guo, T., Zhang, S., Smith, P. P., & Feng, B. (2022). Blocking peripheral drive from colorectal afferents by subkilohertz dorsal root ganglion stimulation. Pain, 163(4), 665-681. https://doi.org/10.1097/j.pain.0000000000002395
Chowdhury, D., Datta, D., & Mundra, A. (2021). Role of Greater Occipital Nerve Block in Headache Disorders: A Narrative Review. Neurol India, 69(Supplement), S228-S256. https://doi.org/10.4103/0028-3886.315993 C
oppola, G., & Schoenen, J. (2012). Cortical excitability in chronic migraine. Curr Pain Headache Rep, 16(1), 93-100. https://doi.org/10.1007/s11916-011-0231-1
Cowan, W. M., Harter, D. H., & Kandel, E. R. (2000). The emergence of modern neuroscience: some implications for neurology and psychiatry. Annu Rev Neurosci, 23, 343-391. https://doi.org/10.1146/annurev.neuro.23.1.343
de Prado, B. M., & Russo, A. F. (2006). CGRP receptor antagonists: A new frontier of antimigraine medications. Drug Discov Today Ther Strateg, 3(4), 593-597. https://doi.org/10.1016/j.ddstr.2006.11.003
Deuis, J. R., Dvorakova, L. S., & Vetter, I. (2017). Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci, 10, 284. https://doi.org/10.3389/fnmol.2017.00284
Divakaran, S., & Loscalzo, J. (2017). The Role of Nitroglycerin and Other Nitrogen Oxides in Cardiovascular Therapeutics. Journal of the American College of Cardiology, 70(19), 2393- 2410. https://doi.org/https://doi.org/10.1016/j.jacc.2017.09.1064
Dodson, H., Bhula, J., Eriksson, S., & Nguyen, K. (2018). Migraine Treatment in the Emergency Department: Alternatives to Opioids and their Effectiveness in Relieving Migraines and Reducing Treatment Times. Cureus, 10(4), e2439. https://doi.org/10.7759/cureus.2439
Dussor, G., Yan, J., Xie, J. Y., Ossipov, M. H., Dodick, D. W., & Porreca, F. (2014). Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci, 5(11), 1085-1096. https://doi.org/10.1021/cn500083e
Edvinsson, J. C. A., Vigano, A., Alekseeva, A., Alieva, E., Arruda, R., De Luca, C., D’Ettore, N., Frattale, I., Kurnukhina, M., Macerola, N., Malenkova, E., Maiorova, M., Novikova, A., Rehulka, P., Rapaccini, V., Roshchina, O., Vanderschueren, G., Zvaune, L., Andreou, A. P., . . . European Headache Federation School of Advanced, S. (2020). The fifth cranial nerve in headaches. J Headache Pain, 21(1), 65. https://doi.org/10.1186/s10194-020-01134-1
Feldman, D., & Scott, K. (2020). Editorial overview: Systems neuroscience. Curr Opin Neurobiol, 64, iii. https://doi.org/10.1016/j.conb.2020.10.011 Goadsby, P. J., Holland, P. R., Martins-Oliveira, M., Hoffmann, J., Schankin, C., & Akerman, S. (2017). Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev, 97(2), 553-622. https://doi.org/10.1152/physrev.00034.2015
Handler, A., & Ginty, D. D. (2021). The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci, 22(9), 521-537. https://doi.org/10.1038/s41583- 021-00489-x
Headache Classification Committee of the International Headache Society, I. (2013). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia, 33(9), 629-808. https://doi.org/10.1177/0333102413485658
Hodge, R. D., Bakken, T. E., Miller, J. A., Smith, K. A., Barkan, E. R., Graybuck, L. T., Close, J. L., Long, B., Johansen, N., Penn, O., Yao, Z., Eggermont, J., Hollt, T., Levi, B. P., Shehata, S. I., Aevermann, B., Beller, A., Bertagnolli, D., Brouner, K., . . . Lein, E. S. (2019). Conserved cell types with divergent features in human versus mouse cortex. Nature, 573(7772), 61-68. https://doi.org/10.1038/s41586- 019-1506-7
Jensen, R., & Stovner, L. J. (2008). Epidemiology and comorbidity of headache. Lancet Neurol, 7(4), 354-361. https://doi.org/10.1016/S1474-4422(08)70062-0
Jones, B. (2021). Why do we need to use animals in neuroscience research? Retrieved April 26 from https://www.eara.eu/post/feature-why-do-we-need-to-use-animals- in-neuroscienceresearch#:~: text=While%20non%2Danimal%20methods%20of,study%20the%20 brain%20in%20action.
Karsan, N., Bose, P., Thompson, C., & Goadsby, P. (2017). PO067 Nitroglycerin triggering as a human migraine model in clinical research. Journal of Neurology, Neurosurgery, and Psychiatry. https://doi.org/10.1136/jnnp-2017-ABN.99
Kendroud, S., Fitzgerald, L. A., Murray, I., & Hanna, A. (2021). Physiology, Nociceptive Pathways. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK470255/
Kim, A. Y., Tang, Z., Liu, Q., Patel, K. N., Maag, D., Geng, Y., & Dong, X. (2008). Pirt, a phosphoinositide-binding protein, functions as a regulatory subunit of TRPV1. Cell, 133(3), 475- 485. https://doi.org/10.1016/j.cell.2008.02.053
Kim, Y. S., Anderson, M., Park, K., Zheng, Q., Agarwal, A., Gong, C., Saijilafu, Young, L., He, S., LaVinka, P. C., Zhou, F., Bergles, D., Hanani, M., Guan, Y., Spray, D. C., & Dong, X. (2016). Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron, 91(5), 1085-1096. https://doi.org/10.1016/j.neuron.2016.07.044
Lipton, R. B., Bigal, M. E., Ashina, S., Burstein, R., Silberstein, S., Reed, M. L., Serrano, D., Stewart, W. F., & American Migraine Prevalence Prevention Advisory, G. (2008). Cutaneous allodynia in the migraine population. Ann Neurol, 63(2), 148- 158. https://doi.org/10.1002/ana.21211
May, A., & Schulte, L. H. (2016). Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol, 12(8), 455-464. https://doi.org/10.1038/nrneurol.2016.93
Moye, L. S., & Pradhan, A. A. A. (2017). Animal Model of Chronic Migraine-Associated Pain. Curr Protoc Neurosci, 80, 9 60 61-69 60 69. https://doi.org/10.1002/cpns.33
Neeb, L., Meents, J., & Reuter, U. (2010). 5-HT(1F) Receptor agonists: a new treatment option for migraine attacks? Neurotherapeutics, 7(2), 176-182. https://doi.org/10.1016/j.nurt.2010.03.003
Niedermirtl, F., Eberhardt, M., Namer, B., Leffler, A., Nau, C., Reeh, P. W., & Kistner, K. (2018). Etomidate and propylene glycol activate nociceptive TRP ion channels. Mol Pain, 14, 1744806918811699. https://doi.org/10.1177/1744806918811699
Nielsen, M. (2020). Advanced Human Anatomy (Seventh ed.). Kendall Hunt. Olesen, J., Burstein, R., Ashina, M., & Tfelt-Hansen, P. (2009). Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol, 8(7), 679-690. https://doi.org/10.1016/S1474-4422(09)70090-0
Opioids and Migraine. (2018). Retrieved March 28 from https://americanheadachesocie¬ty.org/news/opioids-migraine/ Park, J., & Luo, Z. D. (2010). Calcium channel functions in pain processing. Channels (Austin), 4(6), 510-517. https://doi.org/10.4161/chan.4.6.12869
Rindone, J. P., & Sloane, E. P. (1992). Cyanide toxicity from sodium nitroprusside: risks and management. Ann Pharmacother, 26(4), 515-519. https://doi.org/10.1177/106002809202600413
Society, I. H. (2013). The International Classification of Headache Disorders (ICHD-3). Cehpalalgia. https://doi.org/10.1177/0333102413485658
Sureda-Gibert, P., Romero-Reyes, M., & Akerman, S. (2022). Nitroglycerin as a model of migraine: Clinical and preclinical review. Neurobiology of Pain, 12. https://doi.org/https://doi.org/10.1016/j.ynpai.2022.100105
Todd, A. J. (2013). Nociceptive Circuitry in the Spinal Cord. Encyclopedia of Pain. https://doi.org/https://doi.org/10.1007/978-3-642-28753-4_2748
Tortora, G., & Nielsen, M. (2017). Principles of Human Anatomy, John Wiley and Sons.
Trevisani, M., Smart, D., Gunthorpe, M. J., Tognetto, M., Barbieri, M., Campi, B., Amadesi, S., Gray, J., Jerman, J. C., Brough, S. J., Owen, D., Smith, G. D., Randall, A. D., Harrison, S., Bianchi, A., Davis, J. B., & Geppetti, P. (2002). Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci, 5(6), 546-551. https://doi.org/10.1038/nn0602-852
Wattiez, A. S., Gaul, O. J., Kuburas, A., Zorrilla, E., Waite, J. S., Mason, B. N., Castonguay, W. C., Wang, M., Robertson, B. R., & Russo, A. F. (2021). CGRP induces migraine-like symptoms in mice during both the active and inactive phases. J Headache Pain, 22(1), 62. https://doi.org/10.1186/s10194-021-01277-9
Yam, M. F., Loh, Y. C., Tan, C. S., Khadijah Adam, S., Abdul Manan, N., & Basir, R. (2018). General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci, 19(8). https://doi.org/10.3390/ijms19082164
Zheng, Q., Xie, W., Luckemeyer, D. D., Lay, M., Wang, X. W., Dong, X., Limjunyawong, N., Ye, Y., Zhou, F. Q., Strong, J. A., Zhang, J. M., & Dong, X. (2022). Synchronized cluster firing, a distinct form of sensory neuron activation, drives spontaneous pain. Neuron, 110(2), 209-220 e206. https://doi.org/10.1016/j.neuron.2021.10.019

