School of Medicine
61 An examination of the Relationship Between Positive COVID-19 Infection and Vaccine Hesitancy Within the Utah Recover Study Population
Katie Luong and Andrew Phillips (Occupational and Environmental Medicine)
Faculty Mentor: Andrew Phillips (Family & Preventative Medicine, University of Utah)
ABSTRACT
Vaccine hesitancy is defined by the World Health Organization as a “delay in acceptance or refusal of vaccines despite availability of vaccination services,” and is affected by a wide range of factors. Some of these factors identified in the literature include perceived safety and importance of vaccines, as well as high levels of fear regarding the disease in question. This study utilized data from the Utah RECOVER Study (Research on the Epidemiology of SARS-CoV- 2 in Essential Response Personnel), which is a prospective cohort study, to evaluate whether participants’ perception regarding chances of future infection, anxiety regarding future infection, and the importance of obtaining COVID-19 immunizations is changed after being diagnosed with an infection. Using a difference-in-difference calculation, participants who were identified as being COVID-19 positive during the duration of the study did not experience as great an increase in their concern for future COVID-19 infections as did the uninfected group. Furthermore, their concern and anxiety regarding future infections trended towards not increasing as much as those who did not have an infection. By understanding how a history of infection with a particular disease may impact perceptions of future risk and vaccination, approaches toward public health education and campaigns can be modified.
INTRODUCTION
Vaccine hesitancy is defined by the World Health Organization as a “delay in acceptance or refusal of vaccines despite availability of vaccination services,” and is largely affected by a wide range of factors (Jasarevic, 2015). When the COVID-19 vaccine was first introduced in August 2020, herd immunity was an initial public health focus to reduce the impact of the spread of SARS-CoV-2. While the explicit percentage of the population needed to achieve the early goal of herd immunity was not clearly identified, comparisons to diseases such as measles suggested that it would require up to 95% of the population to become vaccinated to reach this goal (Wilder-Smith, 2021).
To date, there have now been over 976 million vaccines administered in the United States, with over 81.4% percent of our entire population now having received at least one dose of the vaccination and only 69.4% having completed a primary series of vaccinations (Centers, 2023b). These vaccination rates fall well short of the early stated immunization goals associated with what was felt to be an opportunity for herd immunity; ultimately, additional investigations in 2022 indicated that herd immunity may not be plausible due to the potential instability of the vaccine and the resulting inability to offer long-term protection to vaccine recipients (Morens, 2022). However, the gap in vaccine uptake despite the far-reaching public health efforts during the early pandemic indicates that vaccine hesitancy was prevalent (World, 2020; Wilder-Smith, 2021).
The Centers for Disease Control has identified at least twelve separate variants that have existed at varying times during the past three years, suggesting that additional variants may continue to emerge (Centers, 2023a). Thus, while recent COVID-19 case numbers and deaths have continued to decline, this ongoing resistance or hesitancy to becoming vaccinated is an ongoing concern for improving the rate of COVID-19 vaccinations (Sekizawa, 2022).
Current literature suggests that safety and importance are major concerns with regard to the reception of vaccines. A study completed in July-August 2020 indicated that 36% of the studied population were reluctant to receive a COVID-19 vaccination, stating that the most frequent reason offered was a “lack of confidence in the safety of the vaccines, followed by lack of confidence in the effectiveness of the vaccine” (Wiysonge, 2022). Concerns regarding safety and importance are not specific to the COVID-19 vaccine, as other vaccines have had hesitancy associated with these issues, however, with regards to COVID-19, this may have been compounded by what has been referred to as the “Covid Infodemic” (Dubé, 2022) or the intensive modern spread of large amounts of information or misinformation that have created doubt about whether a novel vaccine is safe or important, despite the availability of the service.
While the importance of the vaccine, as well as the safety of immunization are significant issues, it is necessary to acknowledge that other factors have been identified as playing a role in vaccine hesitancy during the pandemic. This includes concerns surrounding the efficacy of the COVID-19 infection and/or vaccine, the current dissemination of information in this digital age, socioeconomic status, political climate and/or personal status, and religious and cultural beliefs (Fiselmann, 2022). When delving into the specific topic of infection, the Sekizawa study compared the potential associations between fear of COVID-19 and COVID-19 hesitancy, concluding that those in their study population that identified as having high levels of fear concerning COVID-19 were more willing to receive a vaccine a year later into the study. This result could be attributed to the fact that vaccination is an action that an individual fearful of the virus will take to address their fear.
The Sekizawa study also broke down the fear of COVID-19 into multiple components: a fear of how novel current knowledge is of the virus, how effective or safe the available vaccines actually are, how beneficial currently enacted health measures have been in infection prevention, and a fear of how severe viral infection could be. But the most notable worry is the common concern of a fear of actually becoming infected with the virus (Sekizawa, 2022). Given that fear of being infected appears to be a main driver in leading people to overcome their vaccine hesitancy, another aspect to understand is what, if any, the effect that a person’s own infection has on their views of the vaccine.
Prior infection with COVID-19 has been documented to offer a high level of protection against future reinfection. Various randomized placebo-controlled trials found that COVID-19 vaccinations had a range of 66% to 95% effectiveness in preventing symptomatic COVID-19 infections, a range similar to the 87% protection offered by native immunity. (Pooley, 2023). However, Pooley also found that immunity from both forms of immunity, native or vaccination, wanes over time. This was also illustrated in an additional study conducted in August 2021 that examined a population in Kentucky to compare the likelihood of reinfection between a group who had already been previously infected with COVID-19 and declined vaccination and those who were fully vaccinated. The article found that the unvaccinated, previously infected group, was two times as likely to become reinfected in comparison to those who were fully vaccinated (Cavanaugh, 2021).
Despite such studies suggesting native immunity to be imperfect, other research indicated that a prior COVID-19 infection resulted in a 50% decrease in a participant’s likelihood of receiving a subsequent vaccine (Do, 2022). While prior infection is only one of many diverse variables that can cause an individual to experience vaccine hesitancy, the association between prior infection and vaccine hesitancy is an important subject to investigate as understanding how to improve vaccine uptake in a post-infected population may be critical in protecting from reinfection and subsequent sequelae when native immunity is suboptimal.
In this study, we consider what effect infection with SARS-CoV2 has on participants’ perception regarding the likelihood of future infections, concern regarding future infection, and ultimately, the importance of COVID vaccination. Our hypothesis states that participants who have had at least one positive COVID-19 nasal swab PCR test in the past 9 months are more likely to have a positive perception of vaccinations. While previous literature has stated that there is contrary evidence to this hypothesis, our study collects data beyond the timeframe of prior studies, after additional variants and their resultant spikes of infection occurred. Additionally, with our population being public-facing, essential workers, vaccination would be one of the more important and accessible options for participants to protect themselves against acquiring infection.
METHODS
AN INTRODUCTION TO THE RECOVER STUDY
The RECOVER study is a program funded by the United States Centers for Disease Control (CDC). This program, which was originally initiated in August 2020, is in current collaboration with six different study sites across the United States. These study sites are the University of Miami, in Miami, Florida; St. Luke’s Hospital in Duluth, Minnesota; Baylor Scott and White Health in Temple, Texas; University of Arizona in Tuscon, Arizona; Kaiser Permanente Northwest in Portland, OR; and University of Utah in Salt Lake City, Utah. While the CDC is the source of funding for the project, the RECOVER study design was developed by the CDC in conjunction with Abt Associates, as well as input from investigators at each of the above-listed study sites.
The RECOVER study was initially designed to enroll approximately 3000 essential workers across the 6 study sites and to follow them through the use of active surveillance for at least 18 months. The CDC, Abt Associates, and the investigators at the study sites decided on a number of primary and secondary objectives that ranged from vaccine effectiveness, frequency of SARS-CoV-2 infection and COVID-19 illness, to characterizing the knowledge, attitudes, and practices related to vaccines, and questions regarding immunogenicity among many other questions.
At the start of the study and throughout, medical history, vaccination documentation, and participant demographics were gathered. All participants involved with this study provided online informed consent to indicate their participation. The study protocol and procedures were evaluated and approved by 5 separate IRBs, including the University of Utah. All methods utilized by the study were carried out in observance of current regulations and guidelines.
INCLUSION/EXCLUSION CRITERIA
The eligibility criteria for the RECOVER study consisted of individuals who were either healthcare personnel or frontline workers that had essential jobs that could not be performed from home. One key definition of these essential workers was that they worked at least twenty hours per week and had “direct face-to-face contact, defined as being within 3 feet, or about arm’s length, with co-workers, patients or the public as part of job responsibilities” (University, 2023). An Eligibility Screening Interview was conducted with interested participants to determine those who were eligible. Exclusion criteria included those who do not meet the definition of essential workers, already received a COVID-19 vaccine or had participated in a COVID-19 prevention or treatment investigational trial in the 3 months prior to screening for the RECOVER study.
Specific to this study, participants were selected who had enrolled at the University of Utah site, and answered “No” to the question “Were any of the test results positive or confirmed you were infected?” Infection in the context of the question was the COVID-19 virus. Participants who answered “Yes” to this question were excluded to isolate only the responses of individuals who have never been infected with this particular virus. Participants who did not open the survey or did not provide an answer to the above question were excluded from the data set.
RECRUITMENT
Recruitment was undertaken in phases, with healthcare workers and first responders being the initial group. Later phases included the recruitment of essential frontline workers. Efforts were made to recruit a specific number of participants per occupation, age, and sex stratum at each site. Each site had its own incentive structure; at the University of Utah, participants would receive various amounts of monetary incentives for completing various assigned tasks such as submitting weekly nasal swabs, completing blood draws, or committing to the research study for a year. When the initial study period was coming to a close, the study activities were extended; currently, July 31st, 2023 will be the last day of active participant surveillance and will mark the end of raw data collection for the RECOVER study as a whole.
PARTICIPANT DATA COLLECTION & QUESTIONNAIRE CREATION
The RECOVER study gathers data through three different collection methods: electronic surveys, mid-turbinate nasal swab collections, and blood samples. Text messages, access to medical reports, and emails are used to perform active surveillance. Surveys are sent at various intervals to assess general participant health, usage of personal protective equipment (PPE), frequency of direct contact with other individuals, as well as potential COVID-19 symptoms related to infection or vaccination. Depending upon the survey content, the surveys may be sent weekly, every three months, or after testing positive for COVID-19. Results are collected and organized by RedCap, an online platform that manages online databases and surveys.
In addition to the electronic surveys, on a weekly basis, participants complete at-home, mid-turbinate nasal swab kits. If the participant is having predefined symptoms suggestive of a COVID-illness, an additional anterior nasal dry foam swab, and a saliva sample are also collected. The participants receive assembled kits on a periodic basis from the RECOVER study group, and the completed swabs were sent back to the RECOVER office where research assistants scan and ship the samples directly to a CDC laboratory for reverse transcription polymerase chain reaction (RT-PCR) analysis. Participants are notified of the results of these samples by email on a weekly basis.
Finally, blood samples were collected at enrollment and every 3 months thereafter; additional samples were gathered 28 days after confirmed COVID-19 infection and 14-28 days after a COVID-19 dose. For the University of Utah site, the Clinical & Translational Science Institute (CTSI) assisted our on-site clinical research staff in collecting blood specimens.
Much of the data collected for the broader RECOVER study is not directly relevant to answer the current questions. For this particular examination, surveys sent out every three months from March 31st, 2021 to December 16th, 2022 were utilized for data analysis. This timeframe includes six surveys in total; four of these surveys were used to narrow the amount of analyzed data (Survey 2, Survey 4, Survey 6, and Survey 7); and for the statistical analysis, the earliest and latest survey were used in the calculation. Per each three-month survey, the Utah RECOVER Study sent out 1,181 surveys; Survey 2 received 720 responses, Survey 4 received 480 responses, Survey 6 received 612 responses, and Survey 7 received 605 responses. The specific time periods for each survey are as follows: Survey 2 (March 31, 2021-June 22nd, 2021), Survey 4 (November 17th, 2021-February 15th, 2022), Survey 6 (June 30th, 2022- September 18th, 2022), and Survey 7 (September 22nd, 2022-December 16th, 2022).
Data from nasal PCR swab samples were utilized as opposed to blood serum results. This is secondary to the fact that the PCR swab sample results had a quick turnaround time of 3-5 days in comparison to the delayed response of 3-6 months from blood serum results. Such a delay was felt to make capturing the effect of blood serum results on a participant’s willingness to receive the vaccine more difficult than utilizing the more readily available nasal swab results that participants were asked to complete each week.
OUTCOME VARIABLES
The outcome variables of interest from the above-described surveys were the questions seeking to identify participants’ perceived risk of acquiring COVID-19, anxiety or concern with acquiring COVID-19, and the importance of receiving the COVID-19 vaccine. Specifically, the three separate questions examined were phrased as follows: 1. “Think ahead to the next 6 months. What do you think your chances are of becoming infected or ill with COVID-19?”
1. This question offers a scale of values “0”-“6” or “2”-“8”, with the range of potential answers being almost zero chance, very small chance, small chance, moderate chance, large chance, very large chance, and almost certain. The two different scales of value were a result of different coding designations that occurred during the creation of Survey 2 versus Survey 4. The difference in numerical value was adjusted to account for the same answer range across the four surveys. (It will be identified moving forward as “CovidChance”, which is a reference to the codebook name it was originally designated as during the creation of the survey.)
2. “Think ahead to the next 6 months. How worried are you about becoming infected or ill with COVID-19?”
1. This question offers a scale of values “0”-“4”, with the answer range being not at all worried, a little worried, moderately worried, very worried, and extremely worried. (It will be identified as “CovidWord”.)
3. “How important do you think getting an additional COVID-19 vaccine or booster dose is to protect yourself against COVID-19?” 1. This question offers a scale of values “1”-“5”, with the answer range being not at all important, not too important, somewhat important, very important, and extremely important. (It will be identified as “Booster”.)
The responses to these survey questions were compared against their nasal swab sample PCR results.
DATA ORGANIZATION AND STATISTICAL ANALYSIS
A descriptive analysis was performed to identify how the different components of the participant data set could be broken down based on sex, age group, education, and income level. Furthermore, these participants were identified by whether they had received their first vaccination, as well as if they had been infected at least once with COVID-19 by the end of the study period in December 2022. Participants were asked to report their vaccination status by submitting their vaccination cards and were designated as being vaccinated if they had become vaccinated before (greater than 3 months prior to enrollment) or during their participation in the study.
To identify a change in perception, the original set of data was composed of the numerical answers to the three survey questions, corresponding survey dates when the participant took the survey, and the dates of any identified positive infections. All data from participants who did not receive a PCR-positive result were categorized as “negative”; participants who received a PCR-positive result were categorized as “positive”. To determine a change in perceptions over time, a difference-in-difference was calculated. For both groups, the initial difference calculated was the final survey results (Survey 7) to the initial survey responses (Survey 2). These results were then compared to each other by running a difference-in-difference study, with a p-value of 0.05 being used to determine statistically significant results.
RESULTS
The data for 693 RECOVER participants were identified and gathered. Participant characteristics are summarized in Tables 1 and 2, with Table 1 comparing participants who had received a vaccination or were unvaccinated and Table 2 comparing infected or non-infected participants. Participants included a 1:3 ratio of males to females, 88.89% of participants were between the ages of 18-49, 69.40% had obtained a college degree or an additional graduate or professional degree, and 57.00% were within the income brackets of $25,000-124,999.
When a differences-in-differences analysis was performed, it was determined that the results of CovidChance were statistically significant with a difference-in-difference estimate of -0.325 and a p-value of 0.0478. This indicates that after a participant tested positive for COVID-19, their perception of a future infection did not increase to the same degree as did those that did not have a COVID-19 infection. The resulting model and statistical analysis for CovidChance are in Graph 1 & Table 3.
The result of CovidWord was trending towards statistical significance with a value of -0.227 with a p-value of 0.0744; the model and statistical analysis are in Graph 2 and Table 4. This conclusion indicates that after a participant tested positive for COVID-19, they were potentially less concerned or worried about prospective positive COVID-19 infection in the future than those who did not have a COVID-19 infection. The similarity in numerical value indicates a similar sentiment to the results of CovidChance, but further investigations must be conducted to identify a statistically significant comparison.
The result analysis of Booster was a value of -0.161, with a p-value of 0.530. Because the result of the Booster analysis was not statistically significant, it could not be determined if there was a clear association between a willingness to become vaccinated and a participant’s testing status. To better identify if there is such an association, additional analyses with a more diverse and large population should be conducted. The model and statistical analysis for Booster are found in Graph 3 and Table 5.
Table 1: A demographic comparison of participants who had received a vaccination or were unvaccinated.
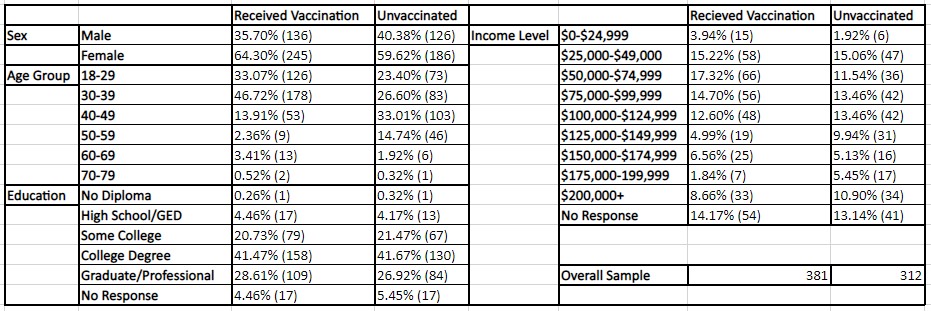
Table 2: A demographic comparison of infected or non-infected participants. Note that the phrase “Infected” represents individuals that have been infected at least once during the examination period
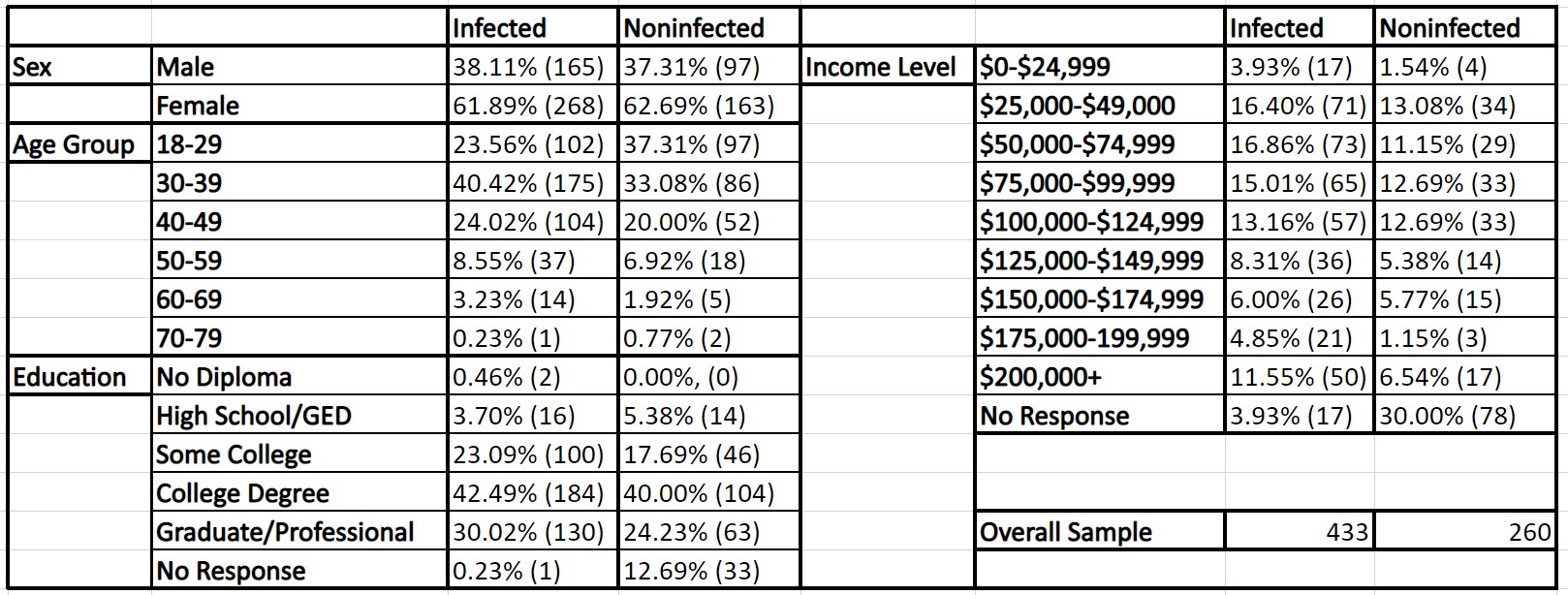
Graph 1: The difference-in-difference model for CovidChance
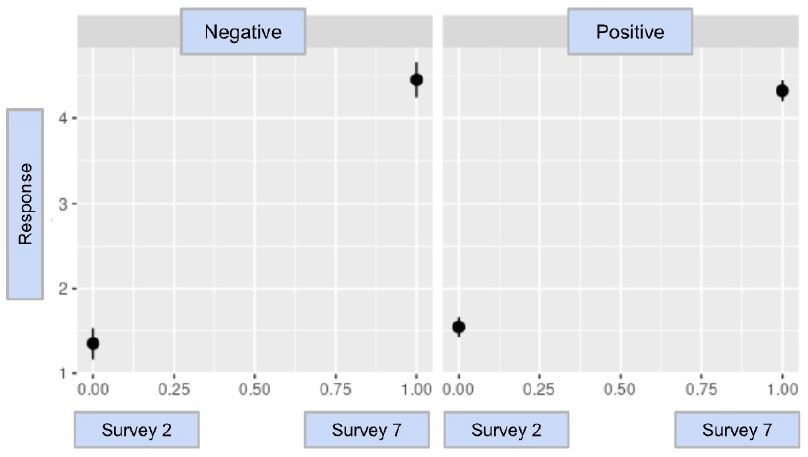
Graph 2: The difference-in-difference model for CovidWord
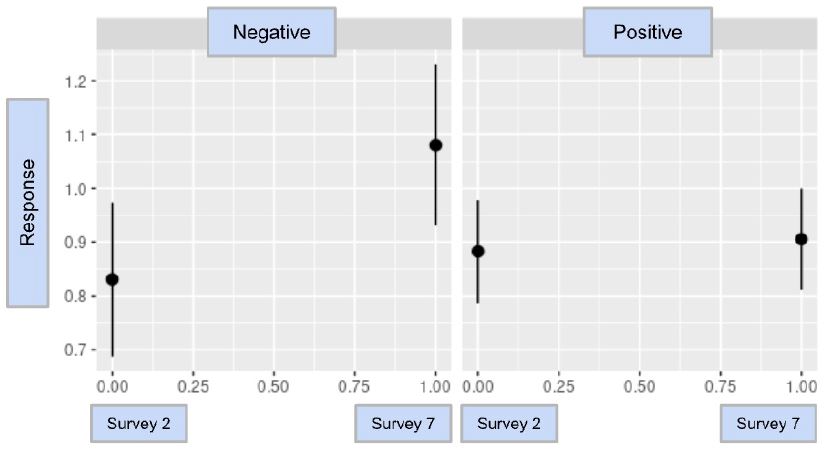
Graph 3: The difference-in-difference model for Booster
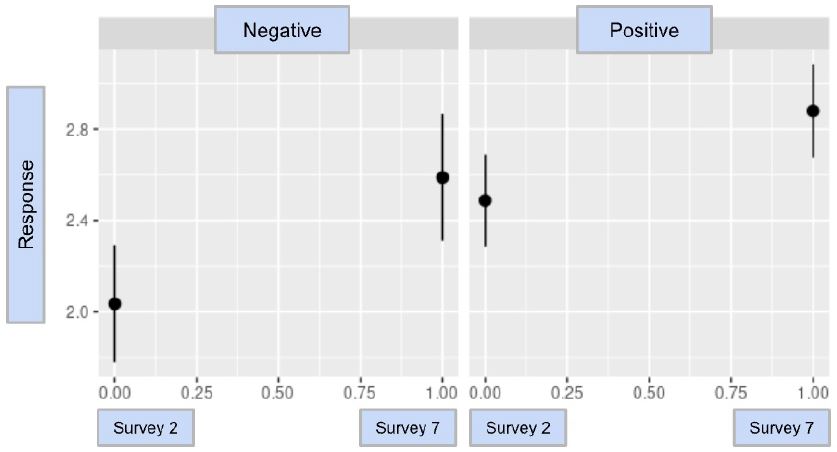
Table 3: The difference-in-difference analysis for CovidChance; the p-value is 0.0478
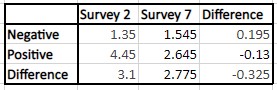
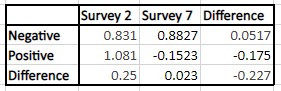
Table 4: The difference-in-difference analysis for CovidWord; the p-value is 0.0744
DISCUSSION
This study examined the relationship between the notification of a positive COVID-19 infection and participant perception of COVID-risk and vaccine hesitancy within a study population of the RECOVER study program. It was determined that during the time period of March 31st, 2021 to December 16th, 2022, participants who tested positive did not have as sharp of an increase in their estimated probability of the potential of testing positive for a second time. Despite the statistical significance, the resulting value of -0.325 indicates that the difference in responses between the two groups may not have much clinical significance.
Given the complexity of vaccine hesitancy noted in the literature, one’s recent COVID-19 status is only one aspect of any person’s perception of the future risk and need for immunization. Participant awareness of information spread by the media at the time could cause a change in perception. At the start of the examination period, a weekly epidemiological update for the week of March 30th, 2021 placed new cases of COVID-19 global infections at nearly 3.8 million (World, 2021); for the week of December 5th, 2022, there were over 3.3 million new cases of infection (World, 2022). This information would have been easily and digitally accessible to any interested individual, including participants in the RECOVER study who were asked to take three-month surveys at this time. With the demographics of this specific study primarily being in the age range of 18-49, and in conjunction with the previously referenced “Covid Infodemic”, it is likely that our participants were aware of the declining rates of infection during this time period.
Furthermore, in terms of the examination time frame, participant perceptions are likely to have been greatly swayed in a significant way due to the introduction of additional COVID-19 variants, such as the Omicron variant. The CDC identified the Omicron variant on November 26th, 2021, which was determined to be the most common variant, replacing the Delta variant. Moreover, the Omicron variant was estimated to be “more predisposed to causing transmission before symptom onset than Delta” (Zeng, 2023). Notably, the identification of the Omicron variant occurred near the halfway point of this study’s study time frame; despite new variants, participants who had a prior infection appeared to feel confident in the native immunity acquired from the infection and felt they had a reduction in risk for a future occurrence. It may be that the presence of a widely publicized new variant decreased the perceived effectiveness of prior native immunity leading to a smaller effect size, however, survey questions were limited in their scope to evaluate such questions.
Given that infections from different pathogens will trigger native immunity of varying levels of effectiveness, it is important to recognize cases where native immunity is inherently ineffective, particularly when there is an available vaccination. In such cases, results seen in this study, as well as in the literature (Do, 2022), indicate that clear messaging needs to be provided to the general population to help them understand the true level of protection of both native immunity as well as that which can be provided by vaccinations.
As noted above, a comparison of the survey results regarding concern or anxiety regarding a future infection did not meet statistical significance, though the p-value was trending towards significance for a lower value in the infected group. In comparison to the difference-in-difference with the other questions, the slope in this analysis visually was different; however, the large confidence intervals likely led to the p-value being non-significant. Given the similarities between the two survey questions (CovidChance and CovidWord), it is not surprising that both results are trending in a similar direction.
It is important to distinguish the fact that there is a wide range of limitations that can cause discrepancies in this particular set of data results. Beyond the direct analysis of blood serum and nasal swab samples, the RECOVER study is dependent on our participants to self-report infections, vaccinations, symptoms, personal information, and much more. Thus, there is an inherent chance that there could be inaccurate reports resulting from participant perception, misunderstanding, reluctance, and/or refusal to share personal information. For example, when participants are asked if they have any potential symptoms of an infection, there are subjective differences in any one individual’s perception as they report their results. In addition, at the most basic level, a participant’s refusal to complete a survey, blood draw, or nasal swab sample will create data inaccuracies. At the height of the pandemic, it was recommended by the Occupational Safety and Health Administration (OSHA) that employers should “instruct any workers who are infected, unvaccinated workers.. and all workers with COVID-19 symptoms to stay home from work” (Occupational, 2021). Hesitancy to report symptoms to the RECOVER Study can thus be potentially linked to a fear of missing work time and subsequent income loss. Finally, while this examination does not use the results of blood serum samples, hesitancy or fear of blood collection can also translate to an unwillingness to complete surveys and weekly nasal swab samples that participants are asked to complete at their own convenience.
In terms of the nasal swab samples, there can be a failure to collect these samples due to the participant or their assigned courier missing the weekly swab collection. A missed swab sample will result in a missed PCR test, which if it was a positive sample, results additionally in the loss of a completed active surveillance form that is sent out to any participant who has either tested positive for COVID-19 or has reported potential symptoms of infection. Furthermore, because a PCR-based test will detect viral genetic material, which can “stay in (the) body for up to 90 days after (testing) positive” (Centers, 2022), the continued weekly testing of nasal swab samples paired with the participant being notified in the same manner will potentially cause a skew in perception even if the participant no longer considered infectious.
The demographics of the study population are not well-balanced in the representation of a normal population, which presents another limitation. An inspection of general RECOVER study participant demographics indicates that the majority of the study population identified themselves to be white and female, with most individuals possessing a college degree or beyond. As a result, this study may not be generalizable to those beyond the demographics represented in this study.
Finally, conducting a differences-in-difference analysis requires that there is a parallel trends assumption for our model. This requires the assumption that if all RECOVER participants had never tested positive for COVID-19, their perceptions concerning vaccine hesitancy would be constant. While there is inherent variability, as noted above there are parallel trends in the data, therefore, the differences-in-differences analysis appears to be an appropriate tool for an examination of the changes in outcome over time within our population.
The strengths of the study include the large population that was involved in the RECOVER, of which the vast majority were engaged with the surveys throughout the duration of the study. While the above limitations of the PCR testing are discussed above, the consistent PCR testing of the participant nasal swab samples provides relatively timely feedback to participants regarding their COVID-19 status, as opposed to other designs which may rely more upon clinical signs and symptoms. Finally, the prospective nature of our study eliminates a number of the biases that may be seen in other, retrospective, or cross-sectional studies, and provides an accurate picture of the change in the participants’ perceptions of these survey questions.
CONCLUSION
This study identified changes in RECOVER participant perception of the importance of vaccine uptake, as well as the risk and severity of future COVID-19 infection. Based upon results from our study, prior infection appears to influence one’s perspective with regards to the likelihood of future infection; the literature suggests that this may be true for a time, but as we have seen over time, new variants and waning immunity may lead to an underestimation of future risk. By contributing to the growing body of literature related to this particular subject, there will be more confidence in improving future medical practices towards vaccination and related education to the general public, with a greater understanding of how differently those with native infections may perceive the need for vaccination compared to those who have yet to be infected. Perception should be studied in the future in regard to other factors such as individual health and pre-existing medical conditions to identify if other aspects contribute to vaccine hesitancy, as well as to help direct public health efforts toward improving vaccination rates.
REFERENCES
Cavanaugh, A. M., Spicer, K. B., Thoroughman, D., Glick, C., & Winter, K. (2021, August 13). Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination – Kentucky, May-June 2021. Morbidity and Mortality Weekly Report. https://pubmed.ncbi.nlm.nih.gov/34383732/
Centers for Disease Control and Prevention. (2022, September 28). COVID-19 Testing: What You Need to Know. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html#:~:te xt=NAATs%2C%20such%20as%20PCR%2Dbased,days%20after%20you%20tes t%20positive.
Centers for Disease Control and Prevention. (2023a, March 20). SARS-CoV-2 Variant Classifications and Definitions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
Centers for Disease Control and Prevention. (2023b, April 12). Covid-19 Vaccinations in the United States. Centers for Disease Control and Prevention. https://covid. cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-count-total
Do, D. P. & Frank, R. (2022). Prior COVID-19 infection: an underappreciated factor in vaccine hesitancy in the USA. Oxford University Press Public Health Emergency Collection. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8807188/
Dubé, E & MacDonald, N. E. (2022). COVID-19 vaccine hesitancy. Nature Reviews Nephrology. 18, 409-410. https://doi.org/10.1038/s41581-022-00571-2
Fieselmann, J., Annac, K., Erdsiek, F., Yilmaz-Aslan, Y., & Brzoska, P. (2022). What are the reasons for refusing a COVID-19 vaccine? A qualitative analysis of social media in Germany. BMC Public Health. 22(846). https://doi.org/10.1186/s12889 -022-13265-y
Jasarevic, T. (2015, August 18). Vaccine hesitancy: A growing challenge for immunization programmes. World Health Organization. https://www.who.int/ news/item/18-08-2015-vaccine-hesitancy-a-growing-challenge-for-immunization-programmes
Morens, D. M., Folkers, G. K., & Fauci, A. S. (2022). The Concept of Classical Herd Immunity May Not Apply to COVID-19. The Journal of Infectious Diseases. 226(2). https://doi.org/10.1093/infdis/jiac109
Occupational Safety and Health Administration. (2021, June 10). Protecting Workers: Guidance on Mitigating and Preventing the Spread of COVID-19 in the Workplace. Occupational Safety and Health Administration. https://www.osha.gov/coronavirus/safework
Pooley, N., Karim, S. S., Combadière, B., Ooi, E., Harris, R. C., Seblain, C., Kisomi, M., & Shaikh, N. (2023). Durability of Vaccine-Induced and Natural Immunity Against COVID-19: A Narrative Review. Infectious Diseases and Therapy. 12(2). https:// doi.org/10.1007/s40121-022-00753-2
Sekizawa, Y., Hashimoto, S., Denda, K., Ochi, S., & So, M. (2022). Association between COVID-19 vaccine hesitancy and generalized trust, depression, generalized anxiety, and fear of COVID-19. BMC Public Health. 22(126). https://doi.org /10.1186/s12889-021-12479-w
University of Utah Health. (2023). Who Can Participate. University of Utah Health. https://medicine.utah.edu/dfpm/occupational-environmental-health/research/recov er/who-can-participate Wilder-Smith, A. (2021). COVID-19 in comparison with other emerging viral diseases: risk of geographic spread via travel. Tropical Diseases, Travel Medicine and Vaccines. 7(3). https://doi.org/10.1186/s40794-020-00129-9
Wiysonge, C. S., Ndwandwe, D., Ryan, J., Jaca, A., Batouré, O., Anya, B, & Cooper, S. (2022). Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Human Vaccines & Immunotherapeutics. 18(1). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8920215/
World Health Organization. (2020, December 31). Coronavirus disease (COVID-19): Herd immunity, lockdowns and COVID-19. World Health Organization. https://www.who.int/news-room/questions-and-answers/item/herd-immunity-lock downs-and-covid-19#:~:text=The%20percentage%20of%20people%20who,amon g%20those%20who%20are%20vaccinated.
World Health Organization. (2021, March 30). Weekly epidemiological update on COVID-19 – 30 March 2021. World Health Organization. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covi d-19—31-march-2021
World Health Organization. (2022, December 14). Weekly epidemiological update on COVID-19 – 14 December 2022. World Health Organization. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covi d-19—14-december-2022#:~:text=Download%20(1.2%20MB)-,Overview,over% 209700%20new%20fatalities%20reported.
Zeng, K., Santhya, S., Soong, A., Malhotra, N., Pushparajah, D., Thoon, K. C., Yeo, B., Ho, Z. J., & Cheng, M. C. (2023). Serial Intervals and Incubation Periods of SARS-CoV-2 Omicron and Delta Variants, Singapore. Emerging Infectious Diseases. 29(4), 814-817. https://doi.org/10.3201/eid2904.220854.

