School of Medicine
48 The Development and Characterization of Biodegradable Paclitaxel-Conjugates for Alzheimer’s Disease (AD) Treatment
Raghad Al-jassimi
Faculty Mentor: Donna Cross (Radiology and Imaging Sciences, University of Utah)
Abstract
Alzheimer’s disease (AD) is a clinical-pathologic condition that is definitively identified at autopsy and 30%-50% of individuals may develop late onset Alzheimer’s Disease (AD) by the age of 85. And although the initial trigger of AD is unknown, the accumulation of amyloid plaques and neurofibrillary tangles has been observed prior to neuronal loss and cognitive decline. However, it is still unclear if amyloid is the cause or an effect of other age-related processes including inflammation, cardiovascular disease, metabolic disorders, etc. In recent years, tau-targeting therapies have been under development, including kinase inhibitors, and tau anti-aggregation molecules. However, many of these strategies have been abandoned due to toxicity and/or ineffectiveness.
Background
Current treatments have focus on amyloid removal, but these have not resulted in measurable cognitive improvements, particularly for late stage of AD. Although these treatments might eventually be beneficial for disease prevention, they are inadequate in the neurodegenerative stage. Treatments studied in our lab have been focused paclitaxel (PTX) and investigating its inability to cross the blood-brain barrier (BBB). PTX has been widely used for treatment of various cancers and has shown promising results in the treatment of AD and TBI, however it does not readily cross the BBB. Conjugation of polymers and peptides to PTX aimed towards solving the issue of BBB impermeability by increasing blood circulation time and chaperoning PTX across the BBB. As a result, we optimized PTX’s therapeutic efficacy using polymer-drug conjugate platform technology, resulting in an effective treatment at significantly lower doses. Overall, our findings suggest that microtubule stabilization via PTX is a promising therapeutic target for both AD and TBI, and that the use of Angiopep-2 peptide and polymer-drug conjugate platform technology could improve its BBB permeability and therapeutic benefits for these disorders.
Methods
Radial Water Maze (RTW) tests were used in this study. To conduct the RTW maze test, mice are placed in the center of the maze and timed for 180s or until they find the escape tube. If a mouse attempts to enter a decoy tube for more than 5s, it is returned to the center of the tub by hand. One the mouse reaches the safety box; it is allowed to remain for 60s and given a treat.
Results
The mice were treated four times with two weeks apart. A two-factor ANOVA revealed a significant difference between groups and a group by days interaction, indicating the benefits of the P-PTX-AP2 conjugate. The Helmert a priori individual comparisons test further confirmed that the P-PTX-AP2-treated group performed significantly better than the saline-treated group and was not significantly different from the wild-type group. The results suggest that the P-PTX-AP2 conjugate is even more effective than intranasal generic PTX in ameliorating cognitive deficits when administered intravenously.
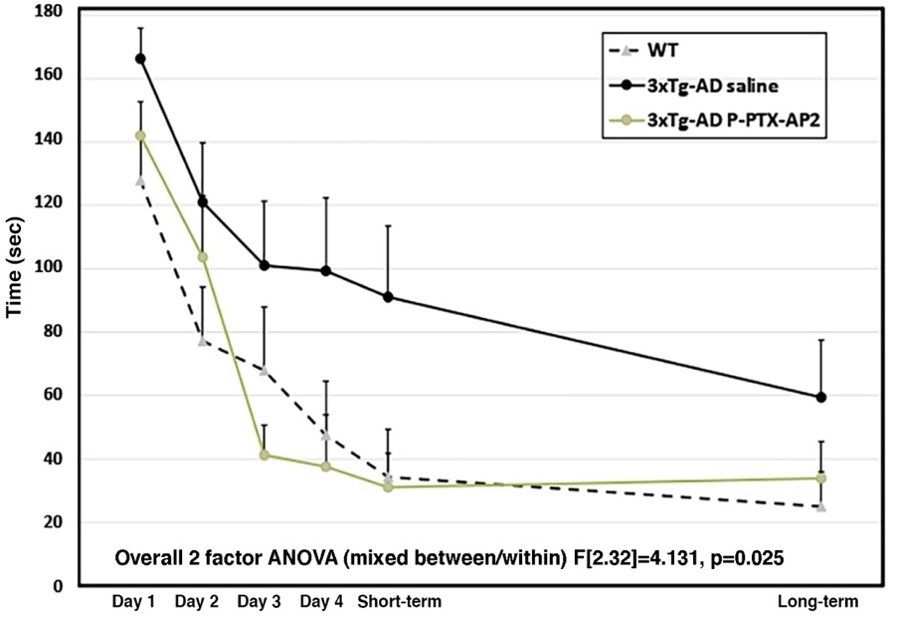
Conclusion
Our study suggests that the P-PTX-AP2 conjugate is effective in ameliorating cognitive deficits in 3xTg-AD mice. This is attributed the AP2 peptide’s ability to facilitate brain uptake and the improved biodistribution of the conjugate. Future studies will require PET Imaging to determine pharmacokinetics and a more thorough outcome evaluation. Our research highlights the potential of low-dose paclitaxel as a promising therapeutic approach for neurodegenerative disorders, particularly Alzheimer’s disease. Furthermore, Alzheimer’s disease remains a significant global health challenge with no available cure. However, our team’s research on the use of low-dose paclitaxel, a microtubule-stabilizing agent, in combination with a brain-targeting peptide, demonstrates potential positive outcomes in preclinical AD models. The novel PTX-conjugates not only improve biodistribution and brain uptake but also exhibit greater efficacy in ameliorating cognitive decline than intranasal generic PTX. Our RWT results showed significat improvement in cognitive ability when comparing treatment stages. This project has high translational potential and could significantly impact the treatment of AD and other CNS conditions such as traumatic brain injury. The availability of a treatment option for these conditions would reduce the enormous suffering experienced by patients and their families and provide a better quality of life for those affected.
Reference
- Jack CR, etal (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alz Dement 14, 535-562.
- Congdon, Erin E, and Einar M Sigurdsson. “Tau-targeting therapies for Alzheimer disease.” Nature reviews. Neurology vol. 14,7 (2018): 399-415. doi:10.1038/s41582-018-0013-z
- Cross DJ, Cook DG et al (2021) Intranasal Paclitaxel Alters Alzheimer’s Disease Phenotypic Features in 3xTg-ADMice. J Alz Dis 83, 379-394
Cross DJ, Meabon JS, Cline MM, Richards TL, Stump AJ, Cross CG, Minoshima S, Banks WA, Cook DG (2019) Paclitaxel Reduces Brain Injury from Repeated Head Trauma in Mice. Journal of Alzheimer’s disease JAD, 2019. 67(3): p. 859-874. - Demeule M, et al, Identification and design of new peptides as a drug delivery system for the brain. J. Pharmacol. Exp. Ther. 324 (2008) 1064–1072.
- Mei L, et al, Angiopep-2 and activatable cell penetrating peptide dual modified nanoparticles for enhanced tumor targeting and penetrating. Int. J. Pharmaceutics 474 (2014) 95-102.
- Kopeček J etal HPMA copolymers: Origins, early developments, present, and future Adv. Drug Delivery Rev. 62 (2010) 122-149. PMC2836498.
- Minko T, Kopeček J, et al Efficacy of chemotherapeutic action of HPMA copolymer-bound Doxorubicin in a solid tumor model of ovarian carcinoma. Int. J. Cancer 86 (2000) 108-117
- Pan H, Yang J, Kopeček J, etal Polymer-drug delivery conjugates and methods of making and using thereof US 9289510 B2 (March 22, 2016).
- Yang J, Kopeček J, et al Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. Reactive Functional Polym. 71 (2011) 294-302.
- Luo K, etal Biodegradable multiblock N-(2-hydroxypropyl)methacrylamide copolymers via reversible addition-fragmentation chain transfer polymerization and click chemistry Macromolecules 44 (2011) 2481-2488 PMC3086388.
- Yang J, Kopeček J, The light at the end of the tunnel – Second generation HPMA conjugates for cancer treatment. Curr. Opin. Colloid Interface Sci. 31 (2017) 30-42. PMC5739067.

