College of Humanities
44 An Activity to Increase Interest in STEM Among Historically Underrepresented Students
Gabrielle Desjardins (University of Utah)
Faculty Mentor: Catherine Goodman (Writing and Rhetoric, University of Utah)
Abstract
In today’s society, occupational opportunities relating to science, technology, engineering, arts, and mathematics (STEM) are increasing. Unfortunately, there is an unequal representation of minorities in STEM-related occupations. In an article written by Jennifer Gunn from Concordia University, she explains that the unequal distribution of people of color in the workplace begins in the grade-school setting. Children of color are less likely to be exposed to STEM in their classrooms, ultimately contributing to their disinterest in pursuing STEM-related careers in the future (Gunn). This lab report outlines an experimental procedure to transform a mixture of milk, vanilla extract, and sugar with rock salt and ice into ice cream. The students will use observational techniques to see that lowering the temperature will cause the mixture to change from a liquid to a solid state. The experiment explores the transition and cause of transition between the three states of matter. The purpose of this lab report is to provide a low-cost activity to instructors and to make STEM more accessible to minority students, so there is an equal opportunity for everyone.
Introduction
A significant number of occupational opportunities originate from the fields of science, technology, engineering, and mathematics (STEM). Unfortunately, there is a gap in the number of minorities in the STEM field. According to Jennifer Gunn from Concordia University, about 70% of the STEM occupation population are white men or women. She attributes this disparity to how minority children are underexposed to STEM in their grade-school years (Gunn). One possibility for underexposure is inadequate funding from the State Board of Education. Without proper funding from the state to perform STEM activities, the teacher would be financially responsible for the entire experiment, and they may not have the resources either. A second reason for underexposure is that the instructors do not have adequate training in STEM. This lab report proposes a way to counteract these challenges by providing explicit instructions for a low-cost experiment. Consequently, instructors can expose their students to STEM in an engaging, fun, and inexpensive way.
An essential concept to grade-school education is the three states of matter. An experiment to turn this concept into an interactive learning experience is to make ice cream using milk, sugar, vanilla extract, ice, rock salt, and plastic bags. The first objective is to educate students on the three states of matter. An applicable example is placing ice (a solid) into a pot on a heated plate. The ice will melt into water (a liquid). Continuing to apply heat will cause the water to boil and emit steam (a gas). The second objective is to hypothesize the effect of reducing the milk’s temperature, which will allow the opportunity for post-experimental discussion as a class. The final objective is to establish a community in the classroom. The students will work as a team throughout the experiment, which will teach them to work together–an essential skill in STEM.
Method
The recipe for the experiment was adapted from the ice cream recipe by Kidzworld and required the following materials: rock salt, skim milk, soy milk, lactose-free skim milk, half and half, white sugar, vanilla extract, ice, a thermometer, measuring cups, 1-gallon and 1-quart plastic bags, spoons, and a small bowl. The instructor prepared the ice cream mixture before the classroom time by combining one cup of milk, two tablespoons of sugar, and one-quarter teaspoon of vanilla extract to a 1-quart plastic bag. The 1-quart plastic bag was sealed airtight, then placed into a second 1-quart plastic bag and sealed.
In the classroom, the experiment followed a short lesson about the states of matter. The lesson was completed by showing a video titled “Bill Nye the Science Guy Phases of Matter,” but could have been completed by a verbal lecture from the instructor. After the lesson, the students washed their hands and cleaned their desks. Next, the students were paired up and given a worksheet (Appendix A) to be completed throughout the experiment. Finally, the instructor guided the students with the following procedure:
- Each pair of students received a spoon, bowl, and a bag containing the prepared mixture. The bag containing the mixture, one scoop of rock
salt, and two cups of ice was placed into a 1-gallon plastic bag. The bag was closed tight to avoid any spills. - A thermometer was used to measure the initial temperature of the mixture (Figure 1) and recorded on the worksheet from Appendix A. At this point, the teacher reminded students to record the temperature and their observations every three minutes on their worksheets.
- The bag was shaken for three minutes, then exchanged by partners. After each three-minute interval, the temperature was measured and recorded.

After four intervals (twelve minutes) passed, the ice cream was frozen and ready for consumption. The students scooped equal portions of the resulting ice cream into two bowls by using the spoon they received at the beginning of the experiment. Every student was involved in the clean-up process. Once everything was clean, the instructor completed the lecture by allowing the students to discuss their results, observations, and examples of the three states of matter.
Results
The results from the experiment were used to create the figures in Appendix B. Figure 2 shows a tabular representation of the recorded temperature at each time interval, and Figure 3 shows a graphical representation of the data for visual comparison. All trials were successfully converter from liquid to solid, with the exception of the soymilk trial. A picture of the final product for the four trials is displayed in Appendix C.
Discussion
The mixture was expected to freeze after ten minutes of shaking; however, at the end of the first trial, the soymilk trial, the mixture was still partially in liquid form. The cause of this was attributed to the ice melting after about six minutes. In an attempt to produce more efficient results, two extra scoops of ice were added to the plastic bag at five minutes on the second trial. Additionally, two minutes were added to the overall time spent shaking the bag. This modification achieved the desired results for the remaining trials.
Another unexpected variation was found in the initial temperatures. The source of this variation coincides with the amount of time each milk was outside of the refrigerator. The soy milk was left at room temperature for the most extended period and had an initial temperature of 7.6 ̊C. The elevated temperature may have contributed to the final product not solidifying completely. The mixture should be kept either in a cooler or a refrigerator until it is given to the children to avoid higher initial temperatures in the future. Then, the mixture should be immediately added to the gallon-size plastic bag with the rock salt and ice. These modifications provided the most desirable outcome.
Conclusion
The experiment demonstrates the states of matter by freezing a liquid mixture of sugar, vanilla extract, and milk using rock salt and ice. Throughout the experiment, the temperatures of each mixture are recorded in three-minute intervals. From this, students learn that reducing the temperature (energy) decreases the motion of the molecules. Although the mixture from the soymilk trial did not freeze completely, the final procedure was adapted to limit the possibility of undesirable outcomes. The purpose of the experiment is to provide an accessible way for minority students to experience STEM. The cost of the ice cream experiment is $16.50 for a class of thirty students. This lab report also provides an explicit, yet simple guideline on how to perform the states of matter experiment. With these tools, children from underrepresented communities are able to explore the field of STEM effectively.
Appendix A: Student Worksheet
Name 1: Name 2:
Complete the drawing:
1. Write the state of matter in the line above the picture.
2. Draw the what the molecules look like in each state of matter in the boxes below. 3. Near the arrows, write the name of the change to get to each state of matter.
Now you are ready to begin your experiment:
A hypothesis is what you think will happen in the experiment. 1. What is your hypothesis for this experiment?
2. Record the temperature of your mixture 3. Record your visual observations here: every three minutes in the table below:

Appendix B: Experimental Data
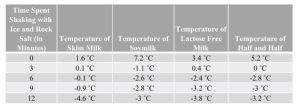
Figure 2: Table of Mixture Temperature Over a Twelve-Minute Shaking Period
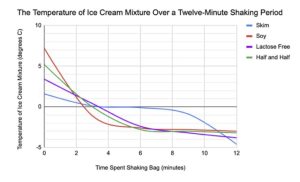
Figure 3: Graph of Mixture Temperature Over a Twelve-Minute Shaking Period
Appendix C: Final Product Appearance
Bibliography
“Bill Nye the Science Guy Phases of Matter.” YouTube, uploaded by Scott Thrope, 3 Feb. 2017, https://www.youtube.com/watch?v=k3SJuozgbfU.
Gunn, Jennifer. “Expanding STEM to Underrepresented Communities.” Room 214, 24 Oct. 2018, Concordia University–Portland, www.education.cu-portland.edu/blog/leaders- link/steam programs-minorities-stem/. Accessed 15 Feb. 2020.
“Science Project: How to Make Homemade Ice Cream.” Kidzworld, 10 July 2019, ww.kidzworld.com/article/26683-science-project-make-homemade-ice-cream. Accessed 10 Feb. 2020.

