College of Social & Behavioral Science
66 Effects of Natural and Urban Imagery on Error-Related Negativity
Marin Macfarlane (University of Utah); Amy McDonnell (Psychology, University of Utah); and David Strayer (University of Utah)
Graduate Student Mentor: Amy S. McDonnell (University of Utah)
Abstract
Attention Restoration Theory (ART) proposes that urban environments deplete our attentional resources and natural environments counteract this depletion by allowing our attentional system to rest and recuperate (Kaplan, 1995). Previous behavioral research supports attention-related benefits of both immersion in nature and viewing images of nature, but little research has utilized brain-imaging to investigate the neural mechanisms underlying these benefits. In the present study, we use electroencephalography (EEG) to investigate the effects of viewing nature imagery in comparison to urban imagery and no imagery on the Error-Related Negativity (ERN), a component of the Event-Related Potential (ERP) related to cognitive control and attention. Previous research has shown an increase in ERN amplitude during immersion in nature compared to immersion in an urban environment, indicative of an increase in cognitive control capacity during immersion in nature. We similarly measured amplitude of the ERN after participants viewed either nature or urban imagery to see if images of nature would have the same effect as immersion in nature does. We predicted an increase in the ERN amplitude for the nature imagery condition compared to the urban imagery condition as well as a no imagery control. However, we found no statistically significant difference in ERN amplitude between the nature and urban imagery conditions, as well as between the nature and no imagery conditions, suggesting that viewing nature imagery may not have the same effect on brain activity as immersion in nature. Future research could investigate whether viewing nature imagery for longer periods of time may be necessary to significantly influence the ERN.
Introduction
In modern society, it is increasingly common for people to spend little time outdoors. Although 75% of American adults value spending time in nature, over 50% spend less than 5 hours outside per week (Kellert et al., 2017). American parents of children ages 8 to 10 report that their children spend 3 times as many hours indoors watching television or playing computer games than they do playing outside (Kellert et al., 2017). Significant barriers to spending time in nature include the allure of technology, indoor obligations such as school or work, and a lack of accessibility to natural environments. By 2050, 70% of Americans are predicted to live in urban environments, making accessibility to natural environments increasingly difficult (Heilig, 2012). These statistics are problematic because certain behavioral and psychological benefits may become compromised due to a lack of access and time in natural environments. Spending time in nature has been shown to reduce depressive symptoms and improve mood (Gidlow et al., 2016), as well as improve attention and performance on various cognitive tasks (Berman et al., 2008; Hartig et al. 2003; Ohly et al., 2016).
Attention is a valuable resource required for successful completion of various cognitive tasks; however, attention is a limited resource and is therefore subject to depletion by attentionally demanding tasks (Baumeister et al., 2018). Attention Restoration Theory (ART) proposes that urban environments, in particular, deplete our attentional resources and that natural environments can counteract this depletion by allowing our attentional system to rest and recuperate (Kaplan, 1995). Numerous studies have supported this theory and shown positive behavioral effects following nature exposure, such as improved cognitive performance on tasks requiring creativity (Atchley, Strayer, & Atchley, 2012), sustained attention (Berman et al., 2008; Hartig et al., 2003), and working memory (Bratman et al., 2015). Although those who have direct, physical immersion in nature typically receive the most benefit (Townsend & Weerasuriya, 2010), there is evidence suggesting that simply viewing images of nature can induce behavioral benefits related to attention (Berman et al., 2008). A study by Berto (2005) found that those who completed an attentionally demanding task, viewed nature imagery, and then completed the task again performed significantly better the second time than those who did not view nature imagery between tasks. Moreover, Ulrich (1979) found that students who viewed nature imagery prior to taking a test reported significantly lower stress levels and greater levels of positive affect than those who did not. Nature imagery has also been shown to reduce anxiety, stress, and irritability in a variety of applied environments, such as hospitals, dementia care facilities, and prisons (Ulrich & Nadkarni, 2009; Bratman et al., 2012; Nadkarni et al., 2017).
Research on the behavioral effects of nature exposure has been accumulating for decades, but little research has utilized brain-imaging to investigate the neurophysiological mechanisms underlying these reported behavioral effects. Bratman et al. (2015) employed fMRI and found reduced activity in the subgenual prefrontal cortex (sgPFC), a brain area associated with depression, after participants completed a nature walk as compared to participants who completed an urban walk. Van Praag et al. (2017) found fMRI results in alignment with ART: participants who listened to nature sounds as compared to urban sounds had increased activity in the default mode network (DMN), which indicates decreased AN activity (Buckner et al., 2008).
In addition to these initial studies exploring neurophysiological responses to nature as measured with fMRI, preliminary research has found evidence of electrophysiological brain responses to nature, measured with EEG (e.g. Aspinall et al., 2015; LoTemplio et al., 2020; McDonnell et al., under review; Scott et al., in prep; Ulrich, 1981). Ulrich (1981) found greater alpha wave amplitude while participants viewed nature imagery as compared to urban imagery, suggesting higher relaxation, alertness, and lower arousal. Another study found that participants taking a nature walk, as compared to participants taking an urban walk, had neurophysiological signals that suggested a meditative state, along with decreased frustration and arousal (Aspinall et al., 2015). Important to the present study, LoTemplio et al. (2020) found an increase in error- related negativity (ERN) amplitude during a 5-day nature trip as compared to pre- and post-trip recordings that took place in an urban environment. The error-related negativity is an event- related potential (ERP) described as a negative deflection in the brain waveform occurring within 100 ms after an individual makes an error on a task. The amplitude of the ERN increases (becomes more negative) as motivation to avoid errors on a task increase (Gehring et al., 1993; Hajcak & Foti, 2008), and it is thought to be positively correlated with cognitive control and indicative of AN activity—such that as attention allocated to the task increases, ERN amplitude increases. The ERN also increases when individuals are rewarded for good performance (Hajcak et al., 2005), and is positively correlated with levels of anxiety (Endrass et al., 2008; Gehring et al., 2000; Hajcak et al., 2003).
LoTemplio et al. (2020) initially hypothesized that there would be a decrease in ERN amplitude when performing a cognitive task during a 5-day camping trip compared to pre- and post-trip recordings, as ERN is thought to be a manifestation of AN activity. Since ART predicts that our AN rests and down-regulates in nature, and previous studies have shown increased ERN is related to anxiety, the ERN was thus predicted to decrease for the nature condition. This prediction aligned with the results of their pilot study, but their follow-up study found a significant increase in ERN amplitude for the nature condition compared to urban conditions, appearing to contradict ART. However, there are potential explanations for this discrepancy. Their pilot study was underpowered, which can lead to less stable results (Button et al., 2013; Szucs & Ioannidis, 2017); additionally, under the framework of ART, it is possible that sufficient time had already been spent in nature and, therefore, cognitive resources were replenished and ready for use at the time of the task (Kaplan, 1995), leading to more attentional resources and thus an increase in ERN amplitude. Large ERN amplitudes are also associated with several abilities necessary for strong performance on cognitive tasks, such as increased working memory capacity (Coleman et al., 2018), desire for accuracy (Gehring et al., 1993), and self-regulation (Legault & Inzlicht, 2013; Potts et al., 2006). LoTemplio et al. (2020) provided compelling evidence in their follow-up study for the effects of nature on ERN amplitude. Because these results were unexpected based on the pilot data, further research needs to be conducted to clarify the effects of nature on human neurophysiology.
In order to further explore the neural processes behind ART and the effects of different types of nature (i.e., immersion compared to viewing images), the present study measures the effects of viewing nature imagery compared to urban imagery on error-related negativity (ERN) amplitude. Based on the results of LoTemplio and colleagues (2020), we hypothesize that ERN amplitude would increase after viewing nature imagery as compared to viewing no imagery or urban imagery, indicating greater cognitive and attentional resources available to allocate to the task. This research will provide further implications about our relationship to nature and how it affects us on a neurophysiological level.
Method
Participants
Participants (N=56) comprised of both University of Utah undergraduate students as well as community volunteers from the greater Salt Lake City area. Participants were selected using convenience sampling through SONA, a participant pool management system, and through flyers
posted around campus as well as throughout the surrounding area. 77% of participants identified as White/Non-Hispanic, 17% identified as Asian, 4% identified as Hispanic/Latino, and 2% identified as Black/African American. Participants were between the ages of 18 and 50 (M = 25.13, SD = 5.46). Participants were compensated with earning either course credits through SONA, or a payment of $70 cash.
Materials
The nature and urban images used in this study were obtained from a past study investigating the effects of nature imagery on behavior (Berman et al., 2008). Participants’ ERN amplitudes were measured using BioPac Systems EEG electrodes while completing the Eriksen and Eriksen (1974) Flanker task, a cognitive task designed to elicit the ERN. In the Flanker task, participants are asked to respond to the central letter in a five-letter sequence of either congruent stimuli, which consists of all identical letters (e.g., SSSSS or HHHHH), or incongruent stimuli, which has a middle letter associated with the opposite response (e.g., SSHSS or HHSHH). The ERN is generated on each trial that the participant makes an error. EEG data was viewed online through the data collection and analysis software AcqKnowledge.
Procedure
Participants came into the lab for three 2-hour testing sessions spaced one week apart. During the EEG setup, participants filled out a series of self-report questionnaires for a different study that will not be reported in the current study. Participants were randomly assigned to either the nature imagery condition or the urban imagery condition, with 28 participants in each condition. Following EEG setup, participants were taken to an outside patio on campus where they completed two cognitive tasks for a different study, followed by the Eriksen and Eriksen (1974) Flanker task used for the current study. Participants completed three testing sessions, with the imagery manipulation at testing session two, consistent with the design of LoTemplio et al. (2020). All three testing sessions were identical, except for the start of the second session, where participants viewed either 10 minutes of nature imagery or urban imagery before completing the Flanker task. During session one and session three, participants were not provided an imagery stimulus, and sat for 10 minutes in front of a concrete wall before beginning any cognitive tasks. Following each testing session, participants returned to the lab to fill out a final self-report questionnaire unrelated to the present study.
EEG Recording and Processing
We used BioPac Systems electrodes to record EEG, placing electrodes at Cz, Fz, and Pz, and additional electrodes on participants’ faces to record electrooculographic (EOG) signals such as blinks and eye movements for later data cleaning. We placed a reference electrode behind the right ear on the mastoid bone and a ground electrode in the middle of the forehead.
EEG data was processed in MatLab with the EEGLab and ERPLab toolboxes (Lopez- Calderon & Luck, 2014). We first downsampled the data to 250 Hz and filtered from 0.1-30 Hz. Next, we identified all the blinks and eye-movements in the data and corrected them using eye- movement correction procedure (EMCP; Gratton et al., 1983). We then identified the timepoints in which a participant made either a correct response or an incorrect response and averaged them together separately to be left with a single waveform of correct responses and another waveform of incorrect responses. We then subtracted the correct brain activity from the incorrect brain activity to be left with the ERN (which is a difference wave of incorrect minus correct).
Analysis Plan
For our first analysis, comparing the effects of viewing nature imagery and no imagery from Session 1 to 2 to 3 on the ERN, we used a linear mixed effects model to control for repeated measures within an individual and for missing data. In our mixed model, Participant ID was included as a random intercept, ERN amplitude was our dependent variable, and Session was our independent variable, which we included as a fixed effect. For our second analysis comparing the effects of viewing nature imagery and urban imagery on ERN amplitude just at Session 2, we used a Welch Two Sample t-Test.
Results
To test the hypothesis that the ERN increases after viewing nature imagery as compared to no imagery, we analyzed ERN amplitude within the nature viewing participants at Session 1 (M=-3.01, SD=2.86), Session 2 (M=-2.79, SD=2.21), and Session 3 (M=-2.72, SD=2.94). The mean amplitudes of the ERN during no imagery (Session 1 and Session 3) and nature imagery (Session 2) are shown in Figure 1. We ran a linear mixed effects model to determine whether the change in ERN amplitude between sessions was statistically significant. The ERN waveforms for Sessions 1, 2, and 3 are shown in Figure 2. There was not a statistically significant change from Session 1 to Session 2 (p=.719), from Session 1 to Session 3 (p=.488), or from Session 2 to Session 3 (p=.728). To test the hypothesis that the ERN increases after viewing nature imagery (M=-2.79, SD=-2.21) as compared to viewing urban imagery (M=-3.84, SD=2.83), we analyzed ERN amplitude between the nature and urban conditions at Session 2. The mean amplitudes of the ERN during nature and urban imagery conditions are shown in Figure 3. We ran a Welch Two Sample t-test to determine whether the change in ERN amplitude between nature and urban imagery conditions was statistically significant. The ERN waveforms for the nature and urban imagery conditions at session 2 are shown in Figure 4. There was no statistically significant difference between ERN amplitudes of the nature condition and the urban condition (t(50.85)=1.548, p=.128). Therefore, both hypotheses in the current study were not supported.
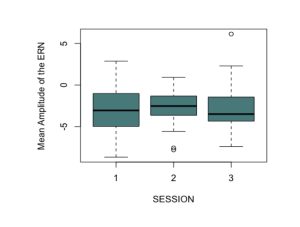
Fig. 1. The mean amplitudes of the error-related negativity (ERN) during no imagery (Session 1 and Session 3) and nature imagery (Session 2) conditions at electrode cite Cz. This shows data from only the nature viewing participants (N=28).
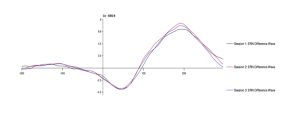
Fig 2. The error-related negativity (ERN) waveform during no imagery (Session 1 and Session 3) and nature imagery (Session 2) conditions at electrode site Cz, where 0 ms is the response onset and the ERN is the negative deflection observed between 0-100ms after response.
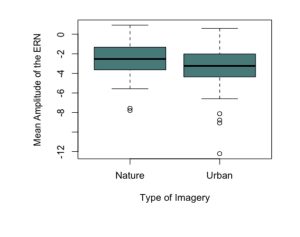
Fig 3. The mean amplitude of the error-related negativity (ERN) during the nature imagery and urban imagery conditions (Session 2) at electrode site Cz.
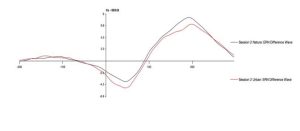
Fig 4. The error-related negativity waveform during the nature imagery and urban imagery conditions (session 2) at electrode site Cz, where 0ms is the response onset and the ERN is the negative deflection observed between 0-100ms after response.
Discussion
The present study investigated the effects of viewing nature imagery, as compared to viewing no imagery or urban imagery, on the ERN amplitude. Previous research has suggested that large ERN amplitudes are associated with greater general cognitive ability (Larson & Clayson, 2011), working memory capacity (Coleman et al., 2018), and motivation to perform tasks well (Boksem et al., 2006). Since nature exposure has been shown to increase performance on various cognitive tasks, particularly those that demand working memory capacity and cognitive control (Ohly et al., 2016), we anticipated that viewing nature imagery prior to completing the Flanker task would increase the ERN amplitude generated by the task. However, our findings did not support this hypothesis, as there was no statistically significant change in ERN amplitude between the nature and no imagery conditions, as well as between the nature and urban imagery conditions.
The findings of the current study suggest that viewing nature imagery has no effect on the ERN. Our null results differ from LoTemplio et al. (2020), who found a statistically significant increase in ERN amplitude during an immersive nature exposure. These divergent findings suggest that nature imagery may not be as powerful as immersion in nature as it relates to influencing the ERN. Although there is evidence to support that direct immersion in nature and indirect exposure to nature, such as viewing nature imagery, have similar behavioral effects, some evidence suggests that direct immersion in nature provides greater benefits (Kaplan, 1993; Kahn et al., 2009). It may be that the neurophysiological effects of nature immersion and viewing nature imagery are also different in this way; that is–the neurophysiological effects of nature immersion are more powerful than the those of indirect exposure to nature. This could contribute to why we did not find a significant change in ERN amplitude for the nature imagery condition.
Limitations & future directions
It is possible that viewing nature imagery for longer periods of time may be necessary to significantly influence the ERN. Based on findings from LoTemplio et al. (2020), the AN may have needed more time to downregulate during nature imagery exposure so it could achieve restoration and therefore greater ERN levels by the time of the Flanker task. At present, the time-course of the behavioral and neural effects of nature is poorly understood. It may be possible that the amount of time needed for nature exposure to induce changes in the ERN is longer than the timeframe used in the current study (i.e., 10 minutes of imagery viewing). Future studies may want to increase the amount of time individuals are exposed to imagery stimulus and compare the effects of different viewing durations on the ERN. There may also be individual differences in the amount of viewing time required to induce changes in the ERN, which we were unable to investigate in the current study; therefore, future research may want to collect enough data to be sufficiently powered for individual difference analyses.
Although the current study did not find statistically significant changes in the ERN as a result of viewing nature imagery, previous work has documented changes in the ERN during nature immersion (LoTemplio et al., 2020). Because of these contrasting findings, it is possible that the benefits of viewing nature imagery may not have the same neural mechanisms as immersion in nature. Further research is needed to clarify the neurophysiological effects of nature exposure, and to determine whether the benefits of nature immersion and viewing nature imagery have similar neural mechanisms. Continuing such research could be an important factor in influencing public policy to protect our natural environments, as they are increasingly threatened by climate change, industrialization, and urbanization. Regarding specifically the nature imagery research, it is important to develop our understanding of its neurophysiological effects as it may have the potential to provide our increasingly urbanized world with greater accessibility to the psychological benefits of nature exposure.
Bibliography
Aspinall, P., Mavros, P., Coyne, R., & Roe, J. (2013). The urban brain: analyzing outdoor physical activity with mobile EEG. British Journal of Sports Medicine, 49(4), 272–276. https://doi.org/10.1136/bjsports-2012-091877
Atchley, R. A., Strayer, D. L., & Atchley, P. (2012). Creativity in the wild: improving creative reasoning through immersion in natural settings. PLoS ONE, 7(12), e51474. https://doi.org/10.1371/journal.pone.0051474
Baumeister, R. F., Bratslavsky, E., Muraven, M., & Tice, D. M. (2018). Ego depletion: is the active self a limited resource?. In Self-regulation and self-control (pp. 16-44). Routledge.
Berman, M. G., Jonides, J., & Kaplan, S. (2008). The cognitive benefits of interacting with nature. Psychological Science, 19(12), 1207–1212. https://doi.org/10.1111/j.1467- 9280.2008.02225.x
Berto, R. (2005). Exposure to restorative environments helps restore attentional capacity. Journal of Environmental Psychology, 25(3), 249–259. https://doi.org/10.1016/j.jenvp.2005.07.001
Boksem, M. A. S., Meijman, T. F., & Lorist, M. M. (2006). Mental fatigue, motivation and action monitoring. Biological Psychology, 72(2), 123–132. https://doi.org/10.1016/j.biopsycho.2005.08.007
Bratman, G. N., Hamilton, J. P., & Daily, G. C. (2012). The impacts of nature experience on human cognitive function and mental health. Annals of the New York Academy of Sciences, 1249(1), 118–136. https://doi.org/10.1111/j.1749-6632.2011.06400.x
Bratman, G. N., Hamilton, J. P., Hahn, K. S., Daily, G. C., & Gross, J. J. (2015). Nature experience reduces rumination and subgenual prefrontal cortex activation. Proceedings of the National Academy of Sciences, 112(28), 8567–8572. https://doi.org/10.1073/pnas.1510459112
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network. Annals of the New York Academy of Sciences, 1124(1), 1–38. https://doi.org/10.1196/annals.1440.011
Button, K. S., Ioannidis, J. P. A., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S. J., & Munafò, M. R. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365–376. https://doi.org/10.1038/nrn3475
Coleman, J. R., Watson, J. M., & Strayer, D. L. (2018). Working memory capacity and task goals modulate error-related ERPs. Psychophysiology, 55(3), e12805. https://doi.org/10.1111/psyp.12805
Endrass, T., Klawohn, J., Schuster, F., & Kathmann, N. (2008). Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia, 46(7), 1877–1887. https://doi.org/10.1016/j.neuropsychologia.2007.12.001
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. https://doi.org/10.3758/bf03203267
Gehring, W. J., Goss, B., Coles, M. G. H., Meyer, D. E., & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. https://doi.org/10.1111/j.1467-9280.1993.tb00586.x
Gehring, W. J., Himle, J., & Nisenson, L. G. (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science, 11(1), 1–6. https://doi.org/10.1111/1467-9280.00206
Gidlow, C. J., Jones, M. V., Hurst, G., Masterson, D., Clark-Carter, D., Tarvainen, M. P., Smith, G., & Nieuwenhuijsen, M. (2016). Where to put your best foot forward: psycho- physiological responses to walking in natural and urban environments. Journal of Environmental Psychology, 45, 22–29. https://doi.org/10.1016/j.jenvp.2015.11.003
Gould van Praag, C. D., Garfinkel, S. N., Sparasci, O., Mees, A., Philippides, A. O., Ware, M., Ottaviani, C., & Critchley, H. D. (2017). Mind-wandering and alterations to default mode network connectivity when listening to naturalistic versus artificial sounds. Scientific Reports, 7(1). https://doi.org/10.1038/srep45273
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology, 55(4), 468-484.
Hajcak, G., & Foti, D. (2008). Errors are aversive. Psychological Science, 19(2), 103–108. https://doi.org/10.1111/j.1467-9280.2008.02053.x
Hajcak, G., McDonald, N., & Simons, R. F. (2003). Anxiety and error-related brain activity. Biological Psychology, 64(1-2), 77–90. https://doi.org/10.1016/s0301-0511(03)00103-0
Hajcak, G., Moser, J. S., Yeung, N., & Simons, R. F. (2005). On the ERN and the significance of errors. Psychophysiology, 42(2), 151–160. https://doi.org/10.1111/j.1469- 8986.2005.00270.x
Hartig, T., Evans, G. W., Jamner, L. D., Davis, D. S., & Gärling, T. (2003). Tracking restoration in natural and urban field settings. Journal of Environmental Psychology, 23(2), 109– 123. https://doi.org/10.1016/s0272-4944(02)00109-3
Heilig, G.K. (2012). World urbanization prospects: the 2011 revision. United Nations, Department of Economic and Social Affairs (DESA), Population Division, Population Estimates and Projections Section, New York, 14, 555.
Kahn Jr, P. H., Severson, R. L., & Ruckert, J. H. (2009). The human relation with nature and technological nature. Current directions in psychological science, 18(1), 37-42.
Kaplan, R. (1993). The role of nature in the context of the workplace. Landscape and urban planning, 26(1-4), 193-201.
Kaplan, S. (1995). The restorative benefits of nature: toward an integrative framework. Journal of Environmental Psychology, 15(3), 169–182. https://doi.org/10.1016/0272- 4944(95)90001-2
Kellert, S. R., Case, D. J., Escher, D., Witter, D. J., Mikels-Carrasco, J., & Seng, P. T. (2017). The nature of Americans: disconnection and recommendations for reconnection. National Report.
Larson, M. J., & Clayson, P. E. (2011). The relationship between cognitive performance and electrophysiological indices of performance monitoring. Cognitive, Affective, & Behavioral Neuroscience, 11(2), 159–171. https://doi.org/10.3758/s13415-010-0018-6
Legault, L., & Inzlicht, M. (2013). Self-determination, self-regulation, and the brain: autonomy improves performance by enhancing neuroaffective responsiveness to self-regulation failure. Journal of Personality and Social Psychology, 105(1), 123–138. https://doi.org/10.1037/a0030426
Lopez-Calderon, J., & Luck, S. J. (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in human neuroscience, 8, 213.
LoTemplio, S. B., Scott, E. E., McDonnell, A. S., Hopman, R. J., Castro, S. C., McNay, G. D., McKinney, T. L., Greenberg, K., Payne, B. R., & Strayer, D. L. (2020). Nature as a potential modulator of the error-related negativity: a registered report. International Journal of Psychophysiology, 156, 49–59. https://doi.org/10.1016/j.ijpsycho.2020.06.014
McDonnell, A.S., LoTemplio, S.B., Scott, E.E., Greenberg, K., McNay, G.D., Castro, S.C., & Strayer, D.L. (In prep). Nature modulates neurophysiological correlates of reward.
Nadkarni, N. M., Hasbach, P. H., Thys, T., Crockett, E. G., & Schnacker, L. (2017). Impacts of nature imagery on people in severely nature-deprived environments. Frontiers in Ecology and the Environment, 15(7), 395–403. https://doi.org/10.1002/fee.1518
Ohly, H., White, M. P., Wheeler, B. W., Bethel, A., Ukoumunne, O. C., Nikolaou, V., & Garside, R. (2016). Attention restoration theory: a systematic review of the attention restoration potential of exposure to natural environments. Journal of Toxicology and Environmental Health, Part B, 19(7), 305–343. https://doi.org/10.1080/10937404.2016.1196155
Potts, G. F., George, M. R. M., Martin, L. E., & Barratt, E. S. (2006). Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neuroscience Letters, 397(1-2), 130–134. https://doi.org/10.1016/j.neulet.2005.12.003
Scott, E.E., McDonnell, A.S., LoTemplio, S.B., McNay, G.D., Greenberg, K., Castro, S.C., & Strayer, D.L. (In prep). Prolonged immersion in nature modulates neural correlates of working memory.
Szucs, D., & Ioannidis, J. P. A. (2017). Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLOS Biology, 15(3), e2000797. https://doi.org/10.1371/journal.pbio.2000797
Townsend, M. and Weerasuriya, R. (2010). Beyond blue to green: the benefits of contact with nature for mental health and well-being. Melbourne, Australia: Beyond Blue Limited.
Ulrich, R. S. (1979). Visual landscapes and psychological well‐being. Landscape Research, 4(1), 17–23. https://doi.org/10.1080/01426397908705892
Ulrich, R. S. (1981). Natural versus urban scenes. Environment and Behavior, 13(5), 523–556. https://doi.org/10.1177/0013916581135001
Ulrich, C., & Nadkarni, N. M. (2009). Sustainability research and practices in enforced residential institutions: collaborations of ecologists and prisoners. Environment, Development and Sustainability, 11(4), 815–832. https://doi.org/10.1007/s10668-008- 9145-4

