39 Use of Isotopes
LumenLearning
Structural Determination
Structural determination using isotopes is often performed using nuclear magnetic resonance spectroscopy and mass spectrometry.
LEARNING OBJECTIVES
Identify the uses of isotopic labeling in structural determination and the primary techniques used to study isotopically-labeled molecules
KEY TAKEAWAYS
Key Points
- Nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS) detect isotopic differences; this allows the position of labeled atoms in a product ‘s structure to be determined.
- Isotopic labeling is a technique used to track the passage of an isotope through a reaction, metabolic pathway, or cell.
- With information about the position of isotopic atoms in products, the reaction pathway can also be determined.
Key Terms
- mass spectrometry: An analytical technique that measures the mass:charge ratio of the ions formed when a molecule or atom is ionized, vaporized, and introduced to a vacuum; may also involve breaking molecules into fragments, enabling structure to be determined.
- nuclear magnetic resonance: The absorption of electromagnetic radiation (radio waves), at a specific frequency, by an atomic nucleus placed in a strong magnetic field; used in spectroscopy and in magnetic resonance imaging.
- isotope: Any of two or more forms of an element where the atoms have the same number of protons but a different number of neutrons; the forms have the same atomic number but a different mass number.
Structural determination utilizing isotopes is often performed using two analytical techniques: nuclear magnetic resonance spectroscopy (NMR) and mass spectrometry (MS).
Nuclear Magnetic Resonance Spectroscopy
NMR analysis is isotope-dependent, and it often relies on trace isotopes of a molecule for detection. For example, the most abundant isotope of carbon, C-12, is invisible to NMR, whereas the minor isotope C-13 is NMR active, but only comprises 1.1 percent of a given sample of carbon. By replacing C-12 in a molecule with C-13, NMR analysis of that position is greatly enhanced. Similarly, H-1 is an NMR active nucleus, whereas H-2 is NMR invisible, so it is possible to determine where a specific hydrogen atom is by replacing it with H-2 and watching for the disappearance of the corresponding signal.
Mass Spectrometry
Mass spectrometry is a technique for determining the molecular weight of an ionized molecule and fragments of the molecule that appear when the molecule is ionized. The addition of an isotope will change the observed mass of the parent ion—the molecule that is ionized and does not fragment. Adding an isotope will also change the observed mass of any fragment which contains the isotope. If a fragment does not contain the isotope, then its mass will not be changed. This can reveal information about the position of an isotope in a molecule.
Isotopic Labeling
Isotopic labeling is used to track the passage of an isotope through a reaction, metabolic pathway, or cell. This leads to information about not only the identity of the reaction product, but also provides information about the mechanism of the reaction or biochemical pathway. This method is commonly used in organic chemistry and biochemistry. The reactant is ‘labeled’ by replacing specific atoms with their isotope. The reaction is allowed to occur and the position of the isotopes in the products is measured; this shows the sequence that the isotopic atom followed during the reaction.
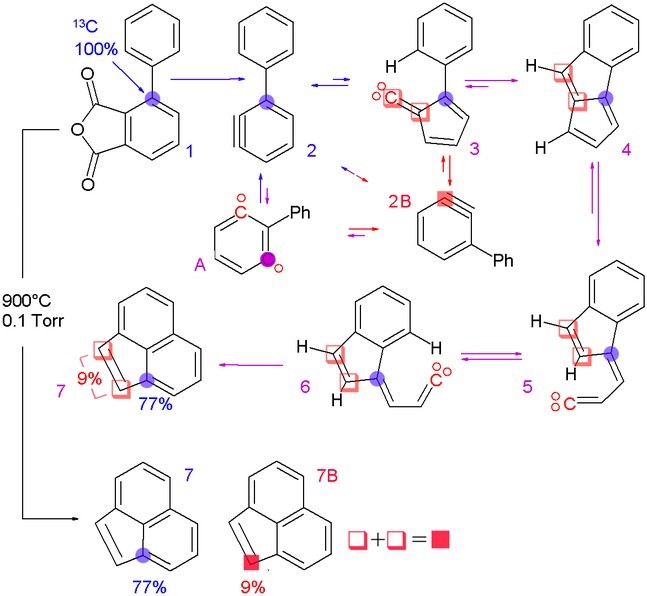
In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes: mass, vibrational mode, or radioactive decay. Mass spectrometry and nuclear magnetic resonance detect the difference in an isotope’s mass, while infrared spectroscopy detects the difference in the isotope’s vibrational modes. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.
An isotopic tracer is used in chemistry and biochemistry to help understand chemical reactions and interactions. In this technique, one or more atoms of a molecule are substituted for a different isotope of the same chemical element. Since the labeled atom—the isotope—has the same number of protons and electrons, it will behave in almost exactly the same way as its unlabeled counterpart. It will not interfere with the reaction being studied. The difference in the number of neutrons, however, means that it can be detected separately from the other atoms of the same element.
Nuclear magnetic resonance and mass spectrometry are used to investigate the mechanisms of chemical reactions. NMR and MS detect isotopic differences; detecting these differences allows information about the position atoms in a product’s structure to be determined. With information about the position of isotopic atoms in products, the reaction pathway can also be determined.
Study of Photosynthesis
Mass spectrometry has been used to study the ratio of carbon isotopes in various plants to understand the mechanisms of photosynthesis.
LEARNING OBJECTIVES
Describe the use of radioactive isotopes in the study of photosynthesis
KEY TAKEAWAYS
Key Points
- The quantities of different isotopes can be measured by mass spectrometry and compared to a standard.
- Mass spectrometry has been used to study the ratio of isotopes in various plants to understand the mechanisms of photosynthesis.
- Plants that follow C3 photosynthetic pathways have ratios of carbon isotopes that differ from those that follow C4 pathways.
Key Terms
- mass spectrometry: An analytical technique that measures the mass:charge ratio of the ions formed when a molecule or atom is ionized, vaporized, and introduced into a vacuum; may also involve breaking molecules into fragments, enabling structure to be determined.
- photosynthesis: The process by which plants and other photoautotrophs generate carbohydrates and oxygen from carbon dioxide, water, and light energy; performed in chloroplasts.
- isotope: Any of two or more forms of an element where the atoms have the same number of protons but a different number of neutrons; forms have the same atomic number but a different mass number.
Isotopes in Photosynthesis
Mass spectrometry has been used to study the ratio of isotopes in various plants to understand the mechanisms of photosynthesis. For example, in laboratory experiments, labeling the atmosphere with oxygen -18 allows us to measure the oxygen uptake by the photorespiration pathway.

Stable carbon isotopes in carbon dioxide are utilized differentially by plants during photosynthesis. C3 carbon fixation is a metabolic pathway that converts carbon dioxide and ribulose bisphosphate into 3-phosphoglycerate. This reaction occurs in all plants as the first step of the Calvin cycle. In C4 plants, carbon dioxide is drawn out of malate and into this reaction rather than being drawn directly from the air.
Grasses in temperate environments, such as barley, rice, and wheat, follow a C3 photosynthetic pathway that yields distinctive isotopic ratios. Grasses in hot, arid environments, specifically maize, but also millet, sorghum, sugar cane, and crabgrass, follow a C4 photosynthetic pathway that produces higher ratios of [latex]^{13}\text{C}[/latex] to [latex]^{12}\text{C}[/latex].
Eating different plants affects the [latex]^{13}\text{C}[/latex] values in the consumer’s body tissues. Case studies show that millet and maize eaters can easily be distinguished from rice and wheat eaters. Studying the geographical distribution of dietary preferences through time can illuminate migration paths of people and dispersal paths of different agricultural crops.
Isotopes in Medicine
Nuclear medicine is a medical specialty that involves the application of radioactive substances to diagnose or treat disease.
LEARNING OBJECTIVES
Describe the use of radioactive isotopes in medicine
KEY TAKEAWAYS
Key Points
- Nuclear medicine is a medical specialty that involves the application of radioactive substances to diagnose or treat disease.
- Nuclear medicine can be used for image physiological functions.
- In addition to imaging, radionuclide therapy can be used to treat conditions such as hyperthyroidism, thyroid cancer, and blood disorders.
- Common isotopes that are used in nuclear imaging include: fluorine-18, gallium-67, krypton-81m, rubidium-82, nitrogen-13, technetium-99m, indium-111, iodine-123, xenon-133, and thallium-201.
Key Terms
- nuclear medicine: The branch of medicine that uses radioactive isotopes in the diagnosis and treatment of disease.
- radiopharmaceutical: Any radioactive substance used as a pharmaceutical.
- physiological: Of, or relating to, the science of the function of living systems.
Nuclear medicine is a medical specialty that involves the application of radioactive substances in the diagnosis and treatment of a disease.
In nuclear medicine procedures, radionuclides are combined with other elements to form chemical compounds. These radiopharmaceuticals, once administered to the patient, can localize to specific organs or cellular receptors. This property of radiopharmaceuticals allows nuclear medicine the ability to image the extent of a disease process in the body. These images are based on cellular function and physiology, rather than on physical changes in the tissue anatomy. Therefore, with some diseases, nuclear medicine studies can identify medical problems at an earlier stage than other diagnostic tests.
Diagnosis
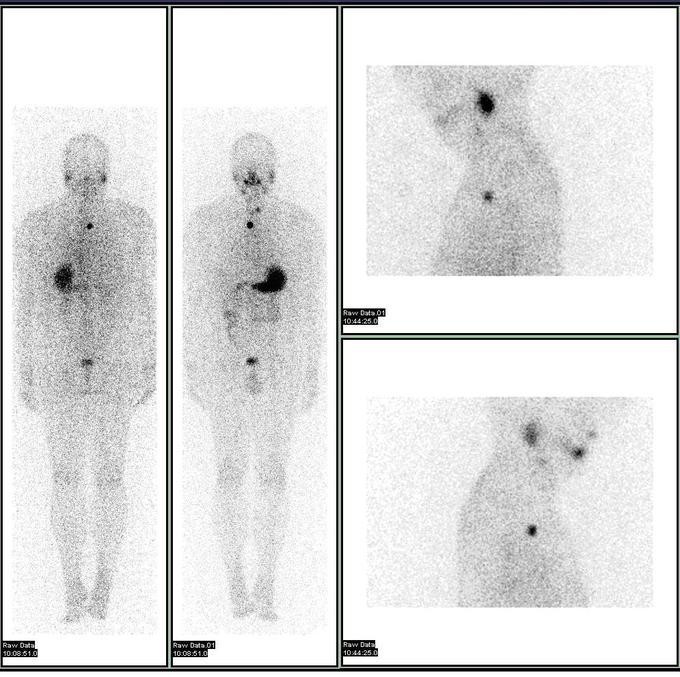
In nuclear medical imaging, radiopharmaceuticals are taken internally, either intravenously or orally. After this, external detectors capture and form images from the radiation that is emitted by the radiopharmaceuticals. This process is unlike a diagnostic X-ray, where external radiation is passed through the body to form an image. Nuclear medicine differs from most other imaging in that diagnostic tests primarily show the physiological function of the system being investigated, as opposed to traditional anatomic imaging, such as CT or MRI.
Common isotopes that are used in nuclear imaging include: fluorine-18, gallium-67, krypton-81m, rubidium-82, nitrogen-13, technetium-99m, indium-111, iodine-123, xenon-133, and thallium-201.
Treatment
In addition to imaging, radionuclide therapy can be used to treat conditions such as hyperthyroidism, thyroid cancer, and blood disorders. The radiopharmaceuticals used in nuclear medicine therapy emit ionizing radiation that travels only a short distance. This thereby minimizes unwanted side effects and damage to noninvolved organs or nearby structures. For this type of therapy, yttrium-90 and iodine-131 are the most commonly used isotopes.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- mass spectrometry. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/mass_spectrometry. License: CC BY-SA: Attribution-ShareAlike
- Isotopic labeling. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Isotopic_labeling. License: CC BY-SA: Attribution-ShareAlike
- isotope. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/isotope. License: CC BY-SA: Attribution-ShareAlike
- nuclear magnetic resonance. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nuclear_magnetic_resonance. License: CC BY-SA: Attribution-ShareAlike
- Isotopic labeling. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Isotopic_labeling. License: Public Domain: No Known Copyright
- Oxygen-18. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Oxygen-18. License: CC BY-SA: Attribution-ShareAlike
- u039413C. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/%CE%9413C. License: CC BY-SA: Attribution-ShareAlike
- Isotopes of carbon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Isotopes_of_carbon. License: CC BY-SA: Attribution-ShareAlike
- C3 carbon fixation. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/C3_carbon_fixation. License: CC BY-SA: Attribution-ShareAlike
- photosynthesis. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/photosynthesis. License: CC BY-SA: Attribution-ShareAlike
- isotope. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/isotope. License: CC BY-SA: Attribution-ShareAlike
- mass spectrometry. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/mass_spectrometry. License: CC BY-SA: Attribution-ShareAlike
- Isotopic labeling. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Isotopic_labeling. License: Public Domain: No Known Copyright
- Photosynthesis. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Photosynthesis. License: Public Domain: No Known Copyright
- Nuclear medicine. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Nuclear_medicine. License: CC BY-SA: Attribution-ShareAlike
- physiological. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/physiological. License: CC BY-SA: Attribution-ShareAlike
- radiopharmaceutical. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/radiopharmaceutical. License: CC BY-SA: Attribution-ShareAlike
- nuclear medicine. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nuclear_medicine. License: CC BY-SA: Attribution-ShareAlike
- Isotopic labeling. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Isotopic_labeling. License: Public Domain: No Known Copyright
- Photosynthesis. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Photosynthesis. License: Public Domain: No Known Copyright
- Nuclear medicine. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Nuclear_medicine. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter "Use of Isotopes" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
Any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei.

