76 Hydrocarbons
OpenStax
LEARNING OBJECTIVES
- Explain the importance of hydrocarbons and the reason for their diversity
- Name saturated and unsaturated hydrocarbons, and molecules derived from them
- Describe the reactions characteristic of saturated and unsaturated hydrocarbons
- Identify structural and geometric isomers of hydrocarbons
The largest database1 of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated2 at 1060—an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds contain only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures. In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons. We can distinguish several types of hydrocarbons by differences in the bonding between carbon atoms. This leads to differences in geometries and in the hybridization of the carbon orbitals.
Alkanes
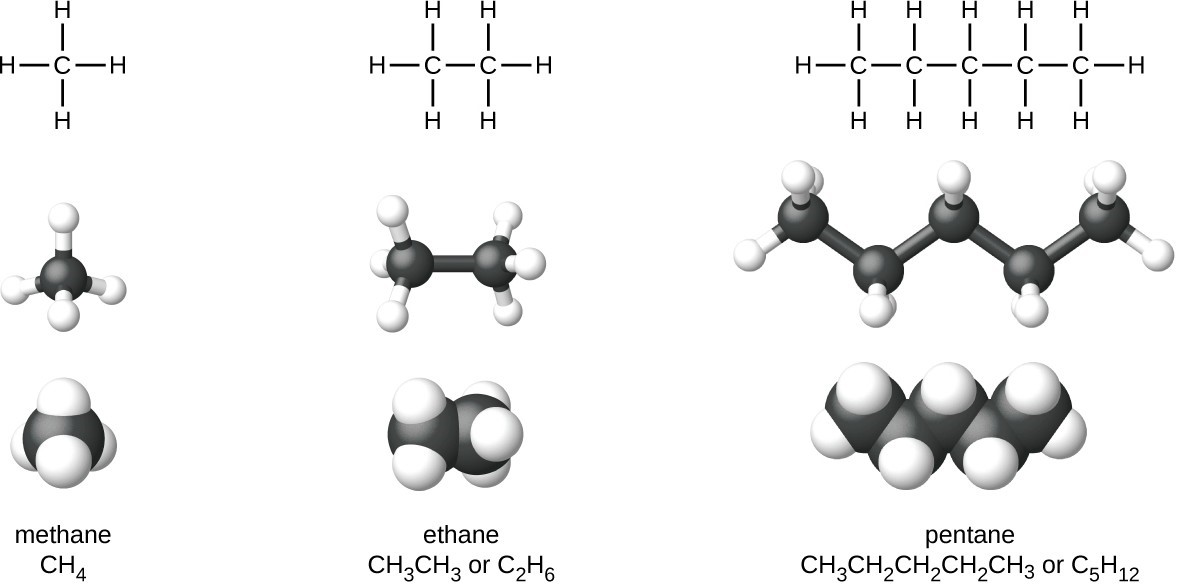
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an alkane has sp3 hybrid orbitals and is bonded to four other atoms, each of which is either carbon or hydrogen. The Lewis structures and models of methane, ethane, and pentane are illustrated in the figure below. Carbon chains are usually drawn as straight lines in Lewis structures, but one has to remember that Lewis structures are not intended to indicate the geometry of molecules. Notice that the carbon atoms in the structural models (the ball-and-stick and space-filling models) of the pentane molecule do not lie in a straight line. Because of the sp3 hybridization, the bond angles in carbon chains are close to 109.5°, giving such chains in an alkane a zigzag shape.
The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas (or simply, condensed formulas). Instead of the usual format for chemical formulas in which each element symbol appears just once, a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a Lewis structure from which most or all of the bond symbols have been removed. Condensed structural formulas for ethane and pentane are shown at the bottom of the figure below, and several additional examples are provided in the exercises at the end of this chapter.
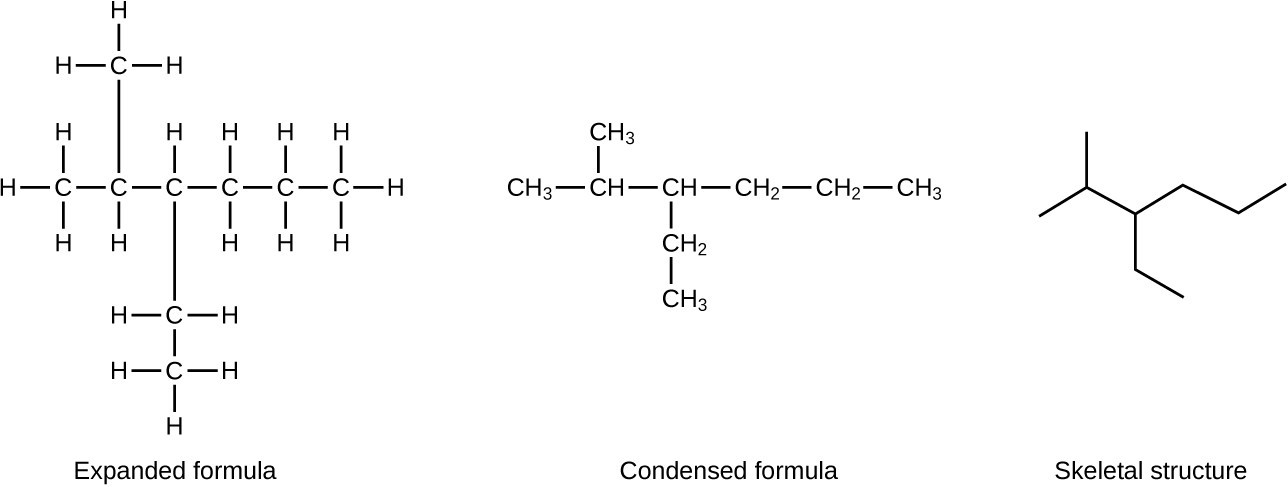
A common method used by organic chemists to simplify the drawings of larger molecules is to use a skeletal structure (also called a line-angle structure). In this type of structure, carbon atoms are not symbolized with a C, but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon. Other atoms besides carbon and hydrogen are represented by their elemental symbols. The following figure shows three different ways to draw the same structure.
EXAMPLE
Drawing Skeletal Structures
Draw the skeletal structures for these two molecules: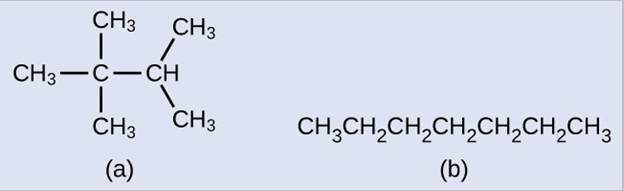
Solution
Each carbon atom is converted into the end of a line or the place where lines intersect. All hydrogen atoms attached to the carbon atoms are left out of the structure (although we still need to recognize they are there):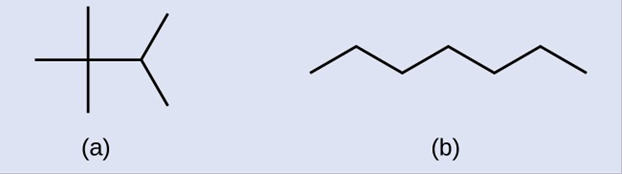
Check Your Learning
Draw the skeletal structures for these two molecules: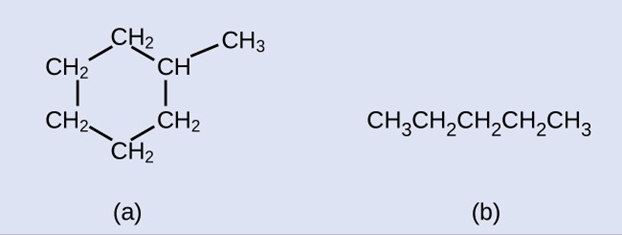
EXAMPLE
Interpreting Skeletal Structures
Identify the chemical formula of the molecule represented here: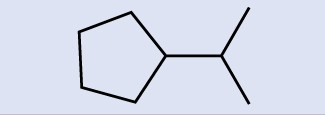
Solution
There are eight places where lines intersect or end, meaning that there are eight carbon atoms in the molecule. Since we know that carbon atoms tend to make four bonds, each carbon atom will have the number of hydrogen atoms that are required for four bonds. This compound contains 16 hydrogen atoms for a molecular formula of [latex]\text{C}_8\text{H}_{16}[/latex].
Location of the hydrogen atoms:
Check Your Learning
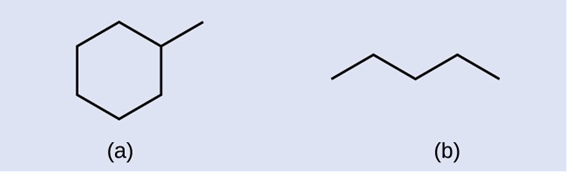
Identify the chemical formula of the molecule represented here: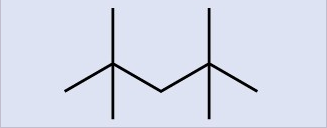
[latex]\text{C}_9\text{H}_{20}[/latex]
All alkanes are composed of carbon and hydrogen atoms, and have similar bonds, structures, and formulas; noncyclic alkanes all have a formula of [latex]\text{C}_\text{n}\text{H}_\text{2n+2}[/latex]. The number of carbon atoms present in an alkane has no limit. Greater numbers of atoms in the molecules will lead to stronger intermolecular attractions (dispersion forces) and correspondingly different physical properties of the molecules. Properties such as melting point and boiling point usually change smoothly and predictably as the number of carbon and hydrogen atoms in the molecules change.
| Properties of Some Alkanes3 | |||||
|---|---|---|---|---|---|
| Alkane | Molecular Formula | Melting Point (°C) | Boiling Point (°C) | Phase at STP4 | Number of Structural Isomers |
| methane | [latex]\text{CH}_4[/latex] | –182.5 | –161.5 | gas | 1 |
| ethane | [latex]\text{C}_2\text{H}_6[/latex] | –183.3 | –88.6 | gas | 1 |
| propane | [latex]\text{C}_3\text{H}_8[/latex] | –187.7 | –42.1 | gas | 1 |
| butane | [latex]\text{C}_4\text{H}_{10}[/latex] | –138.3 | –0.5 | gas | 2 |
| pentane | [latex]\text{C}_5\text{H}_{12}[/latex] | –129.7 | 36.1 | liquid | 3 |
| hexane | [latex]\text{C}_6\text{H}_{14}[/latex] | –95.3 | 68.7 | liquid | 5 |
| heptane | [latex]\text{C}_7\text{H}_{16}[/latex] | –90.6 | 98.4 | liquid | 9 |
| octane | [latex]\text{C}_8\text{H}_{18}[/latex] | –56.8 | 125.7 | liquid | 18 |
| nonane | [latex]\text{C}_9\text{H}_{20}[/latex] | –53.6 | 150.8 | liquid | 35 |
| decane | [latex]\text{C}_{10}\text{H}_{22}[/latex] | –29.7 | 174.0 | liquid | 75 |
| tetradecane | [latex]\text{C}_{14}\text{H}_{30}[/latex] | 5.9 | 253.5 | solid | 1858 |
| octadecane | [latex]\text{C}_{18}\text{H}_{38}[/latex] | 28.2 | 316.1 | solid | 60,523 |
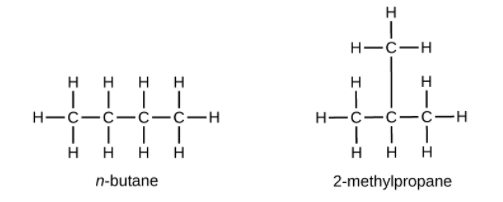
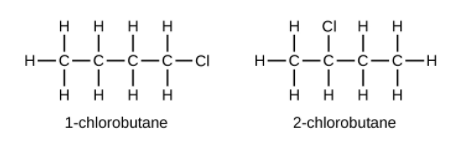
Hydrocarbons with the same formula, including alkanes, can have different structures. For example, two alkanes have the formula [latex]\text{C}_4\text{H}_{10}[/latex]: They are called n-butane and 2-methylpropane (or isobutane), and have the following Lewis structures: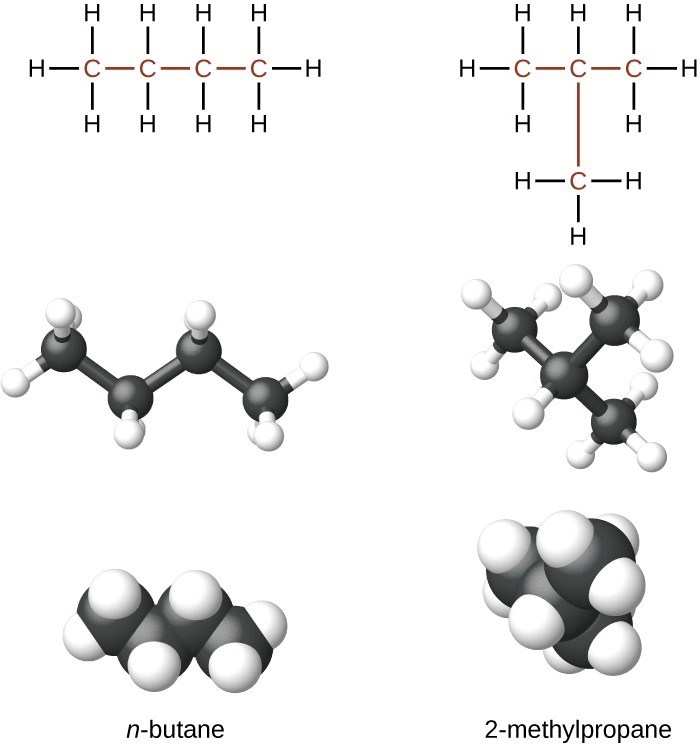
The compounds n-butane and 2-methylpropane are structural isomers (the term constitutional isomers is also commonly used). Constitutional isomers have the same molecular formula but different spatial arrangements of the atoms in their molecules. The n-butane molecule contains an unbranched chain, meaning that no carbon atom is bonded to more than two other carbon atoms. We use the term normal, or the prefix n, to refer to a chain of carbon atoms without branching. The compound 2–methylpropane has a branched chain (the carbon atom in the center of the Lewis structure is bonded to three other carbon atoms)
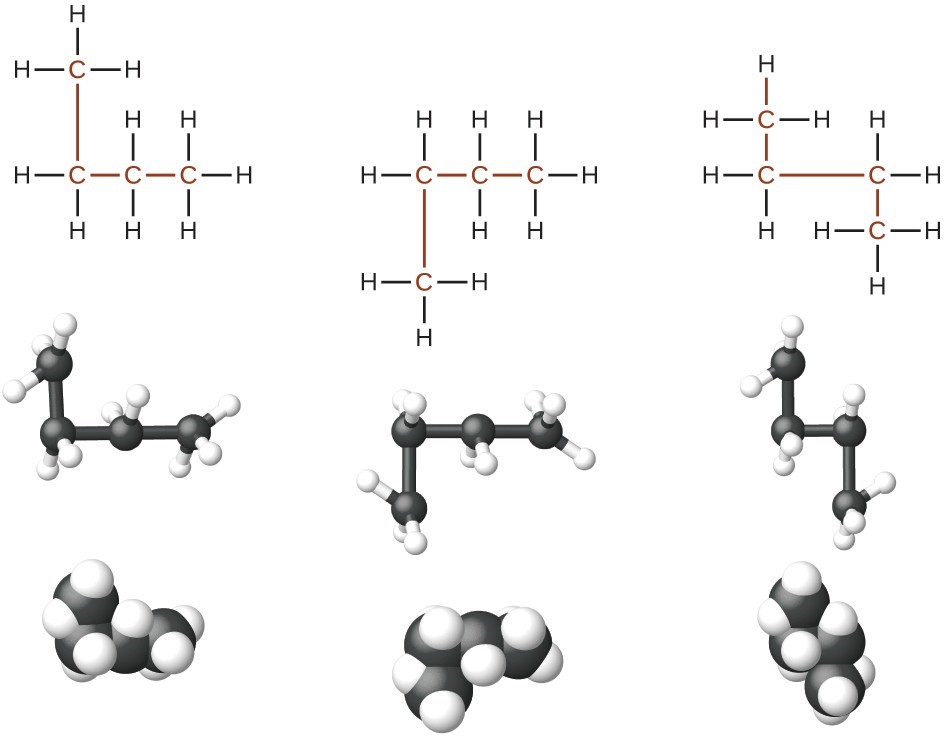
Identifying isomers from Lewis structures is not as easy as it looks. Lewis structures that look different may actually represent the same isomers. For example, the three structures in the figure below all represent the same molecule, n-butane, and hence are not different isomers. They are identical because each contains an unbranched chain of four carbon atoms.
The Basics of Organic Nomenclature: Naming Alkanes
The International Union of Pure and Applied Chemistry (IUPAC) has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures. The nomenclature for alkanes is based on two rules:
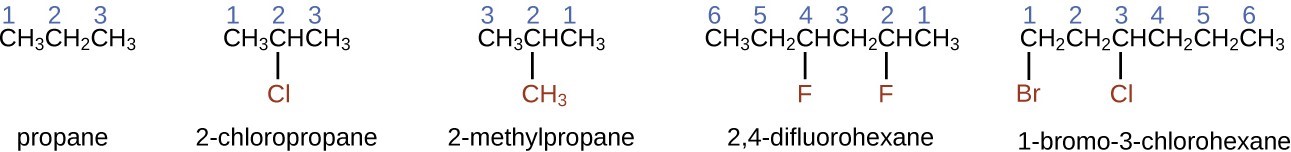
- To name an alkane, first identify the longest chain of carbon atoms in its structure. A two-carbon chain is called ethane; a three-carbon chain, propane; and a four-carbon chain, butane. Longer chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10). These prefixes can be seen in the names of the alkanes described in the figure below.
- Add prefixes to the name of the longest chain to indicate the positions and names of substituents. Substituents are branches or functional groups that replace hydrogen atoms on a chain. The position of a substituent or branch is identified by the number of the carbon atom it is bonded to in the chain. We number the carbon atoms in the chain by counting from the end of the chain nearest the substituents. Multiple substituents are named individually and placed in alphabetical order at the front of the name.
When more than one substituent is present, either on the same carbon atom or on different carbon atoms, the substituents are listed alphabetically. Because the carbon atom numbering begins at the end closest to a substituent, the longest chain of carbon atoms is numbered in such a way as to produce the lowest number for the substituents. The ending -o replaces -ide at the end of the name of an electronegative substituent (in ionic compounds, the negatively charged ion ends with -ide like chloride; in organic compounds, such atoms are treated as substituents and the -o ending is used). The number of substituents of the same type is indicated by the prefixes di- (two), tri- (three), tetra- (four), and so on (for example, difluoro- indicates two fluoride substituents).
EXAMPLE
Naming Halogen-substituted Alkanes
Name the molecule whose structure is shown here: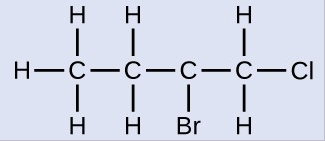
Solution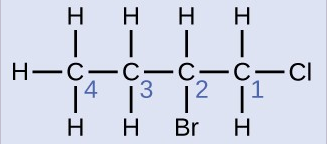
The four-carbon chain is numbered from the end with the chlorine atom. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions 3 and 4). Four carbon atoms means that the base name of this compound will be butane. The bromine at position 2 will be described by adding 2-bromo-; this will come at the beginning of the name, since bromo- comes before chloro- alphabetically. The chlorine at position 1 will be described by adding 1-chloro-, resulting in the name of the molecule being 2-bromo-1-chlorobutane.
Check Your Learning
Name the following molecule: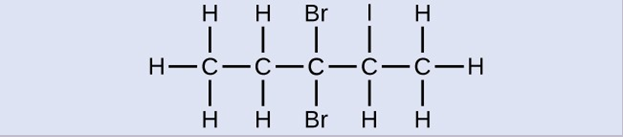
3,3-dibromo-2-iodopentane
We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. The name of an alkyl group is obtained by dropping the suffix -ane of the alkane name and adding -yl: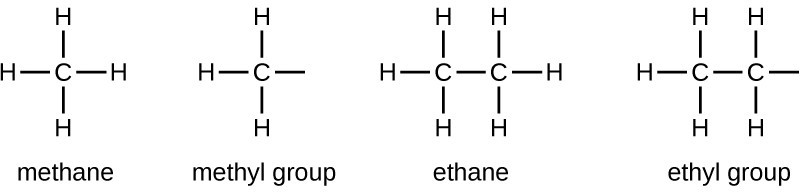
The open bonds in the methyl and ethyl groups indicate that these alkyl groups are bonded to another atom.
EXAMPLE
Naming Substituted Alkanes
Name the molecule whose structure is shown here: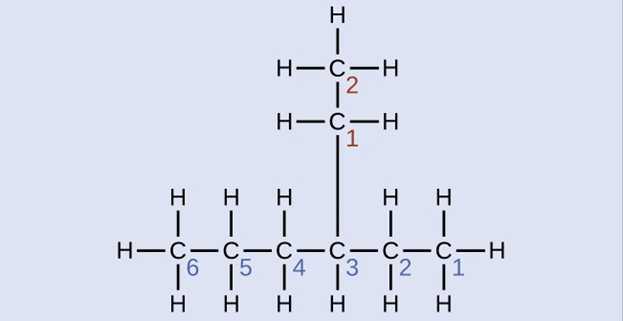
Solution
The longest carbon chain runs horizontally across the page and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch). In this case, we want to number from right to left (as shown by the blue numbers) so the branch is connected to carbon 3 (imagine the numbers from left to right—this would put the branch on carbon 4, violating our rules). The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our name for two carbons eth- and attach -yl at the end to signify we are describing a branch. Putting all the pieces together, this molecule is 3-ethylhexane.
Check Your Learning
Name the following molecule: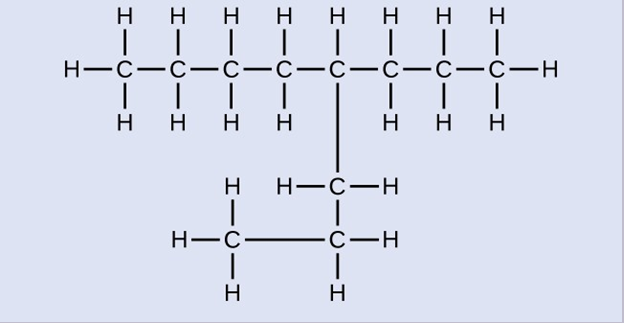
4-propyloctane
Some hydrocarbons can form more than one type of alkyl group when the hydrogen atoms that would be removed have different “environments” in the molecule. This diversity of possible alkyl groups can be identified in the following way: The four hydrogen atoms in a methane molecule are equivalent; they all have the same environment. They are equivalent because each is bonded to a carbon atom (the same carbon atom) that is bonded to three hydrogen atoms. (It may be easier to see the equivalency in the ball and stick models in the figure below. Removal of any one of the four hydrogen atoms from methane forms a methyl group. Likewise, the six hydrogen atoms in ethane are equivalent and removing any one of these hydrogen atoms produces an ethyl group. Each of the six hydrogen atoms is bonded to a carbon atom that is bonded to two other hydrogen atoms and a carbon atom. However, in both propane and 2–methylpropane, there are hydrogen atoms in two different environments, distinguished by the adjacent atoms or groups of atoms: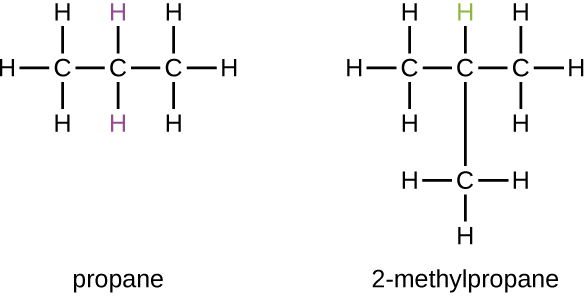
Each of the six equivalent hydrogen atoms of the first type in propane and each of the nine equivalent hydrogen atoms of that type in 2-methylpropane (all shown in black) are bonded to a carbon atom that is bonded to only one other carbon atom. The two purple hydrogen atoms in propane are of a second type. They differ from the six hydrogen atoms of the first type in that they are bonded to a carbon atom bonded to two other carbon atoms. The green hydrogen atom in 2-methylpropane differs from the other nine hydrogen atoms in that molecule and from the purple hydrogen atoms in propane. The green hydrogen atom in 2-methylpropane is bonded to a carbon atom bonded to three other carbon atoms. Two different alkyl groups can be formed from each of these molecules, depending on which hydrogen atom is removed. The names and structures of these and several other alkyl groups are listed in the following table.
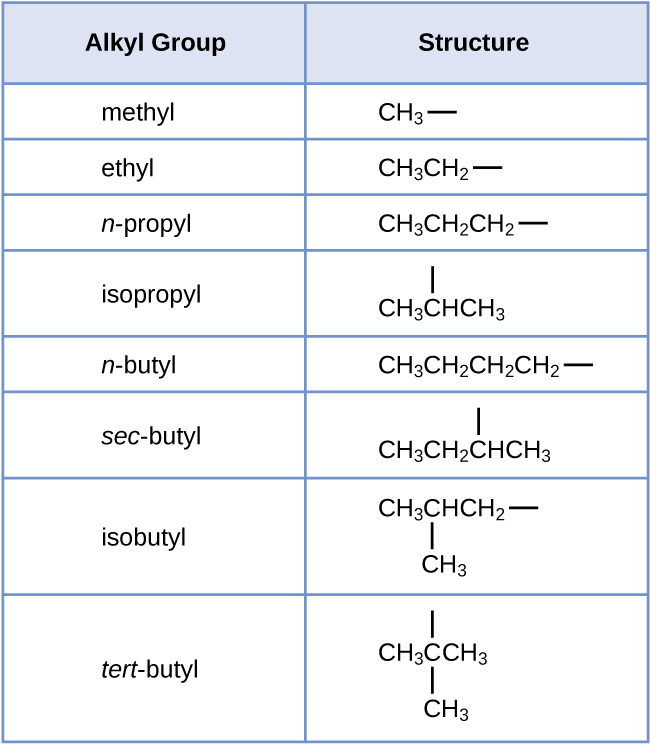
Note that alkyl groups do not exist as stable independent entities. They are always a part of some larger molecule. The location of an alkyl group on a hydrocarbon chain is indicated in the same way as any other substituent: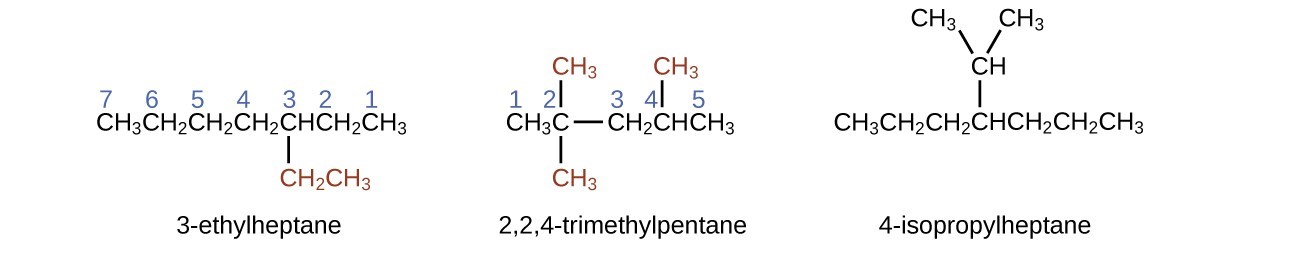
Alkanes are relatively stable molecules, but heat or light will activate reactions that involve the breaking of C–H or C–C single bonds. Combustion is one such reaction:
[latex]\text{CH}_4 (g) + \text{2O}_2 (g) \rightarrow \text{CO}_2 (g) + \text{2H}_2\text{O} (g)[/latex]
Alkanes burn in the presence of oxygen, a highly exothermic oxidation-reduction reaction that produces carbon dioxide and water. As a consequence, alkanes are excellent fuels. For example, methane, [latex]\text{CH}_4[/latex], is the principal component of natural gas. Butane, [latex]\text{C}_4\text{H}_{10}[/latex], used in camping stoves and lighters is an alkane. Gasoline is a liquid mixture of continuous- and branched-chain alkanes, each containing from five to nine carbon atoms, plus various additives to improve its performance as a fuel. Kerosene, diesel oil, and fuel oil are primarily mixtures of alkanes with higher molecular masses. The main source of these liquid alkane fuels is crude oil, a complex mixture that is separated by fractional distillation. Fractional distillation takes advantage of differences in the boiling points of the components of the mixture. You may recall that boiling point is a function of intermolecular interactions, which was discussed in the chapter on solutions and colloids.
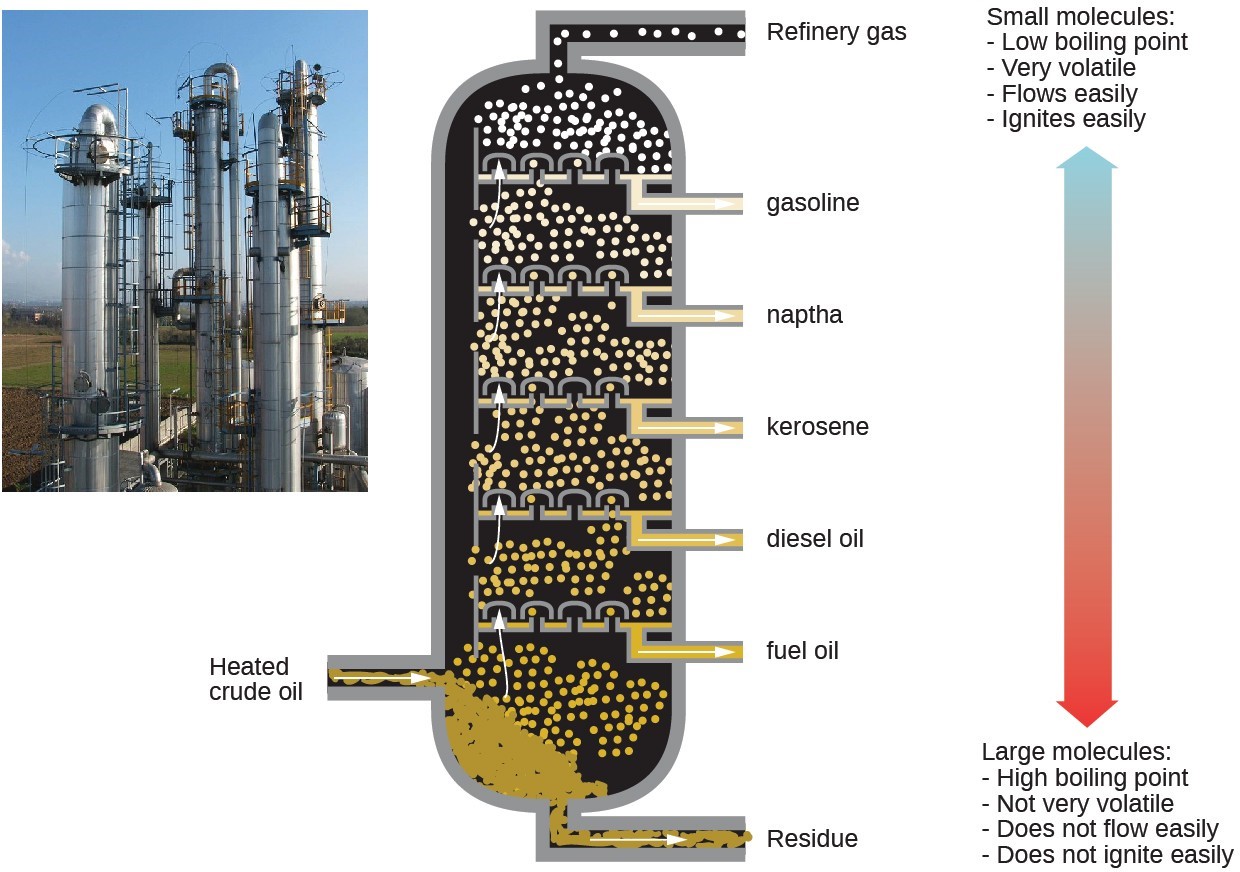
In a substitution reaction, another typical reaction of alkanes, one or more of the alkane’s hydrogen atoms is replaced with a different atom or group of atoms. No carbon-carbon bonds are broken in these reactions, and the hybridization of the carbon atoms does not change. For example, the reaction between ethane and molecular chlorine depicted here is a substitution reaction: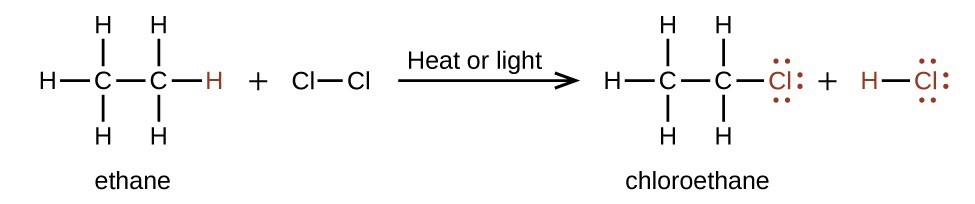
The C–Cl portion of the chloroethane molecule is an example of a functional group, the part or moiety of a molecule that imparts a specific chemical reactivity. The types of functional groups present in an organic molecule are major determinants of its chemical properties and are used as a means of classifying organic compounds as detailed in the remaining sections of this chapter.
Alkenes
Organic compounds that contain one or more double or triple bonds between carbon atoms are described as unsaturated. You have likely heard of unsaturated fats. These are complex organic molecules with long chains of carbon atoms, which contain at least one double bond between carbon atoms. Unsaturated hydrocarbon molecules that contain one or more double bonds are called alkenes. Carbon atoms linked by a double bond are bound together by two bonds, one σ bond and one π bond. Double and triple bonds give rise to a different geometry around the carbon atom that participates in them, leading to important differences in molecular shape and properties. The differing geometries are responsible for the different properties of unsaturated versus saturated fats.
Ethene, [latex]\text{C}_2\text{H}_4[/latex], is the simplest alkene. Each carbon atom in ethene, commonly called ethylene, has a trigonal planar structure. The second member of the series is propene (propylene); the butene isomers follow in the series. Four carbon atoms in the chain of butene allows for the formation of isomers based on the position of the double bond, as well as a new form of isomerism.
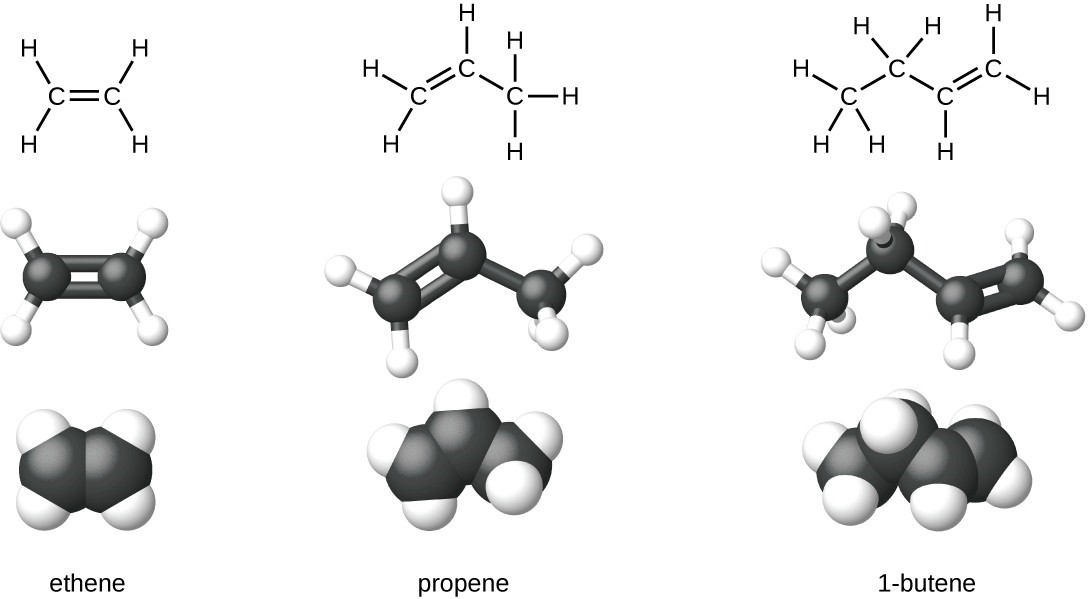
Ethylene (the common industrial name for ethene) is a basic raw material in the production of polyethylene and other important compounds. Over 135 million tons of ethylene were produced worldwide in 2010 for use in the polymer, petrochemical, and plastic industries. Ethylene is produced industrially in a process called cracking, in which the long hydrocarbon chains in a petroleum mixture are broken into smaller molecules.
Chemistry in Everyday Life
Recycling Plastics
Polymers (from Greek words poly meaning “many” and mer meaning “parts”) are large molecules made up of repeating units, referred to as monomers. Polymers can be natural (starch is a polymer of sugar residues and proteins are polymers of amino acids) or synthetic [like polyethylene, polyvinyl chloride (PVC), and polystyrene]. The variety of structures of polymers translates into a broad range of properties and uses that make them integral parts of our everyday lives. Adding functional groups to the structure of a polymer can result in significantly different properties (see the discussion about Kevlar later in this chapter).
An example of a polymerization reaction is shown in the figure below. The monomer ethylene [latex]\text{(C}_2\text{H}_4)[/latex] is a gas at room temperature, but when polymerized, using a transition metal catalyst, it is transformed into a solid material made up of long chains of [latex]\text{–CH}_2–[/latex] units called polyethylene. Polyethylene is a commodity plastic used primarily for packaging (bags and films).
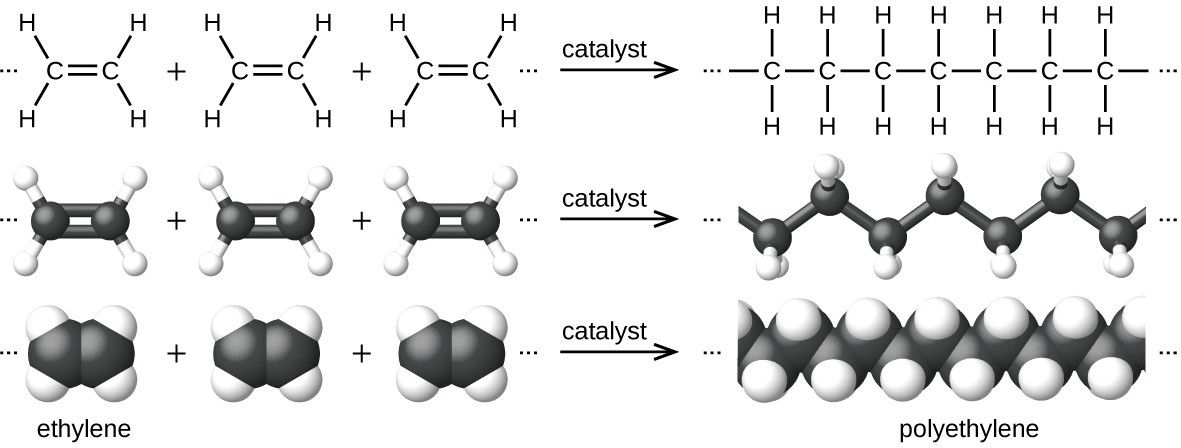
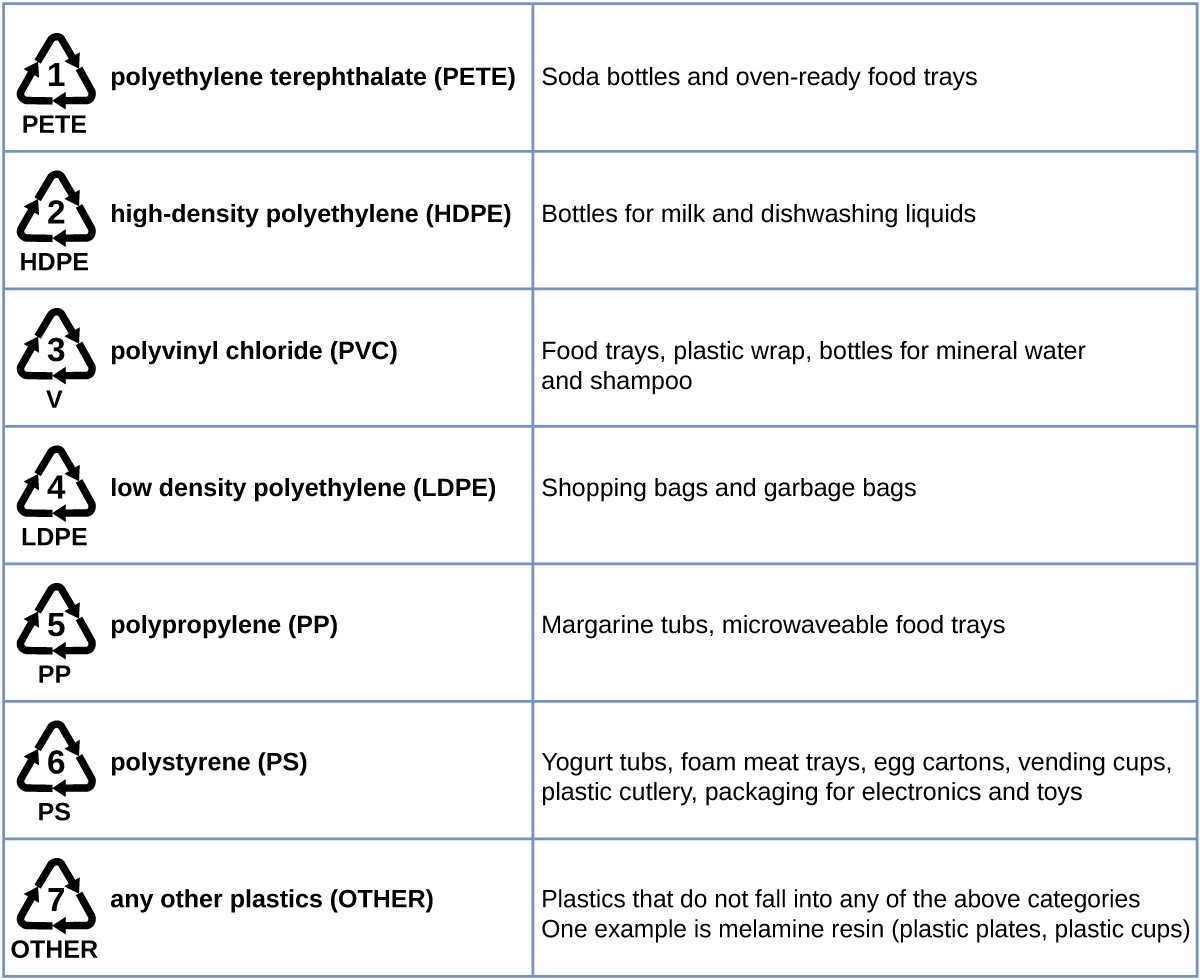
Polyethylene is a member of one subset of synthetic polymers classified as plastics. Plastics are synthetic organic solids that can be molded; they are typically organic polymers with high molecular masses. Most of the monomers that go into common plastics (ethylene, propylene, vinyl chloride, styrene, and ethylene terephthalate) are derived from petrochemicals and are not very biodegradable, making them candidate materials for recycling. Recycling plastics helps minimize the need for using more of the petrochemical supplies and also minimizes the environmental damage caused by throwing away these nonbiodegradable materials.
Plastic recycling is the process of recovering waste, scrap, or used plastics, and reprocessing the material into useful products. For example, polyethylene terephthalate (soft drink bottles) can be melted down and used for plastic furniture, in carpets, or for other applications. Other plastics, like polyethylene (bags) and polypropylene (cups, plastic food containers), can be recycled or reprocessed to be used again. Many areas of the country have recycling programs that focus on one or more of the commodity plastics that have been assigned a recycling code. These operations have been in effect since the 1970s and have made the production of some plastics among the most efficient industrial operations today.
The name of an alkene is derived from the name of the alkane with the same number of carbon atoms. The presence of the double bond is signified by replacing the suffix -ane with the suffix -ene. The location of the double bond is identified by naming the smaller of the numbers of the carbon atoms participating in the double bond: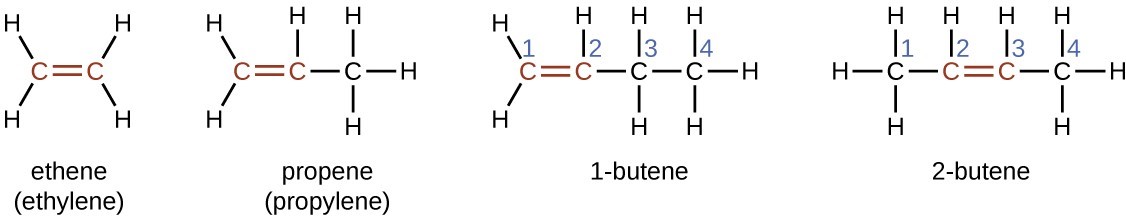
Isomers of Alkenes
Molecules of 1-butene and 2-butene are structural isomers; the arrangement of the atoms in these two molecules differs. As an example of arrangement differences, the first carbon atom in 1-butene is bonded to two hydrogen atoms; the first carbon atom in 2-butene is bonded to three hydrogen atoms.
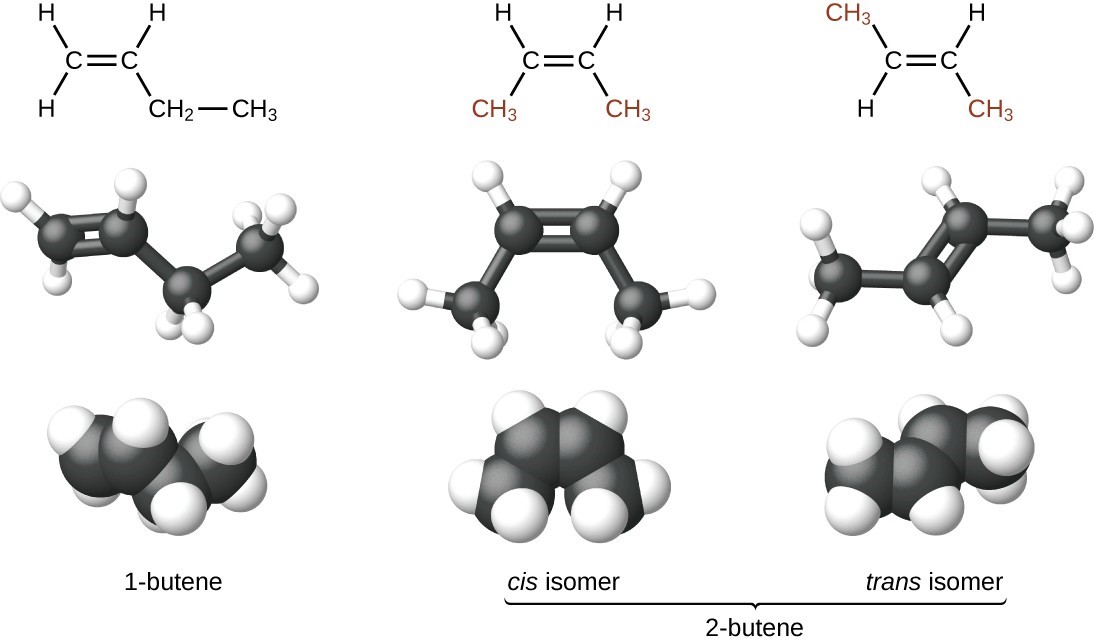
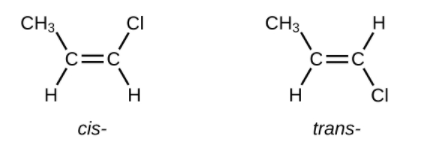
The compound 2-butene and some other alkenes also form a second type of isomer called a geometric isomer. In a set of geometric isomers, the same types of atoms are attached to each other in the same order, but the geometries of the two molecules differ. Geometric isomers of alkenes differ in the orientation of the groups on either side of a [latex]\text{C = C}[/latex] bond.
Carbon atoms are free to rotate around a single bond but not around a double bond; a double bond is rigid. This makes it possible to have two isomers of 2-butene, one with both methyl groups on the same side of the double bond and one with the methyl groups on opposite sides. When structures of butene are drawn with 120° bond angles around the sp2-hybridized carbon atoms participating in the double bond, the isomers are apparent. The 2-butene isomer in which the two methyl groups are on the same side is called a cis-isomer; the one in which the two methyl groups are on opposite sides is called a trans-isomer. The different geometries produce different physical properties, such as boiling point, that may make separation of the isomers possible:
Alkenes are much more reactive than alkanes because the [latex]\text{C = C}[/latex] moiety is a reactive functional group. A π bond, being a weaker bond, is disrupted much more easily than a σ bond. Thus, alkenes undergo a characteristic reaction in which the π bond is broken and replaced by two σ bonds. This reaction is called an addition reaction. The hybridization of the carbon atoms in the double bond in an alkene changes from sp2 to sp3 during an addition reaction. For example, halogens add to the double bond in an alkene instead of replacing hydrogen, as occurs in an alkane: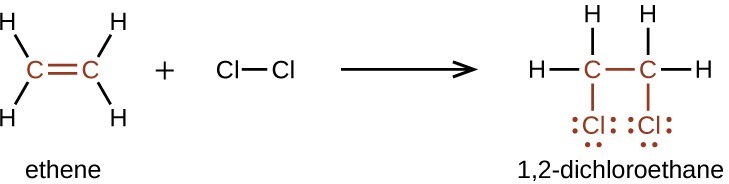
EXAMPLE
Alkene Reactivity and Naming
Provide the IUPAC names for the reactant and product of the halogenation reaction shown here: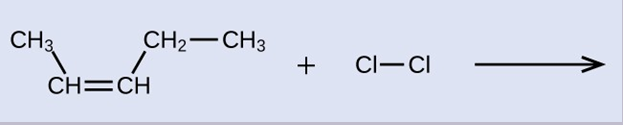
Solution
The reactant is a five-carbon chain that contains a carbon-carbon double bond, so the base name will be pentene. We begin counting at the end of the chain closest to the double bond—in this case, from the left—the double bond spans carbons 2 and 3, so the name becomes 2-pentene. Since there are two carbon-containing groups attached to the two carbon atoms in the double bond—and they are on the same side of the double bond—this molecule is the cis-isomer, making the name of the starting alkene cis-2-pentene. The product of the halogenation reaction will have two chlorine atoms attached to the carbon atoms that were a part of the carbon-carbon double bond: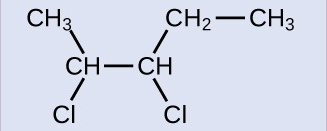
This molecule is now a substituted alkane and will be named as such. The base of the name will be pentane. We will count from the end that numbers the carbon atoms where the chlorine atoms are attached as 2 and 3, making the name of the product 2,3-dichloropentane.
Check Your Learning
Provide names for the reactant and product of the reaction shown: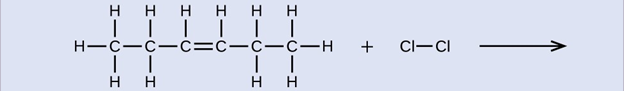
reactant: cis-3-hexene product: 3,4-dichlorohexane
Alkynes
Hydrocarbon molecules with one or more triple bonds are called alkynes; they make up another series of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond are bound together by one σ bond and two π bonds. The sp-hybridized carbons involved in the triple bond have bond angles of 180°, giving these types of bonds a linear, rod-like shape.
The simplest member of the alkyne series is ethyne, [latex]\text{C}_2\text{H}_2[/latex], commonly called acetylene. The Lewis structure for ethyne, a linear molecule, is: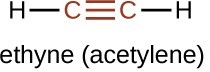
The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. For example, [latex]\text{CH}_3\text{CH}_2\text{C} \equiv \text{CH}[/latex] is called 1-butyne.
EXAMPLE
Structure of Alkynes
Describe the geometry and hybridization of the carbon atoms in the following molecule: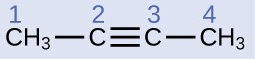
Solution
Carbon atoms 1 and 4 have four single bonds and are thus tetrahedral with sp3 hybridization. Carbon atoms 2 and 3 are involved in the triple bond, so they have linear geometries and would be classified as sp hybrids.
Check Your Learning
Identify the hybridization and bond angles at the carbon atoms in the molecule shown: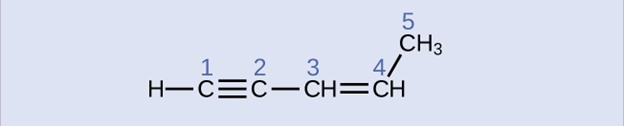
carbon 1: sp, 180°; carbon 2: sp, 180°; carbon 3: sp2, 120°; carbon 4: sp2, 120°; carbon 5: sp3, 109.5°
Chemically, the alkynes are similar to the alkenes. Since the [latex]\text{C} \equiv \text{C}[/latex] functional group has two π bonds, alkynes typically react even more readily, and react with twice as much reagent in addition reactions. The reaction of acetylene with bromine is a typical example: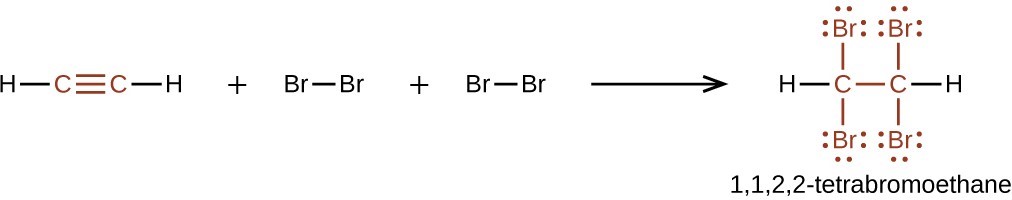
Acetylene and the other alkynes also burn readily. An acetylene torch takes advantage of the high heat of combustion for acetylene.
Aromatic Hydrocarbons
Benzene, [latex]\text{C}_6\text{H}_6[/latex], is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. These compounds contain ring structures and exhibit bonding that must be described using the resonance hybrid concept of valence bond theory or the delocalization concept of molecular orbital theory. (To review these concepts, refer to the earlier chapters on chemical bonding). The resonance structures for benzene, [latex]\text{C}_6\text{H}_6[/latex], are: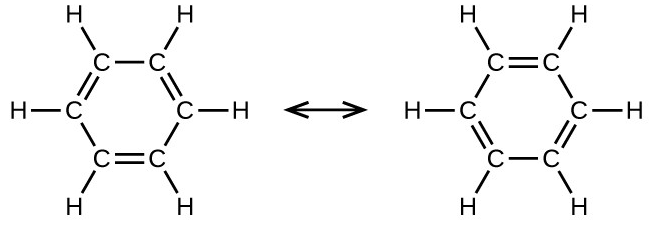
Valence bond theory describes the benzene molecule and other planar aromatic hydrocarbon molecules as hexagonal rings of sp2-hybridized carbon atoms with the unhybridized p orbital of each carbon atom perpendicular to the plane of the ring. Three valence electrons in the sp2 hybrid orbitals of each carbon atom and the valence electron of each hydrogen atom form the framework of σ bonds in the benzene molecule. The fourth valence electron of each carbon atom is shared with an adjacent carbon atom in their unhybridized p orbitals to yield the π bonds. Benzene does not, however, exhibit the characteristics typical of an alkene. Each of the six bonds between its carbon atoms is equivalent and exhibits properties that are intermediate between those of a [latex]\text{C–C}[/latex] single bond and a [latex]C = C[/latex] double bond. To represent this unique bonding, structural formulas for benzene and its derivatives are typically drawn with single bonds between the carbon atoms and a circle within the ring as shown in the following figure.
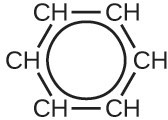
There are many derivatives of benzene. The hydrogen atoms can be replaced by many different substituents. Aromatic compounds more readily undergo substitution reactions than addition reactions; replacement of one of the hydrogen atoms with another substituent will leave the delocalized double bonds intact. The following are typical examples of substituted benzene derivatives: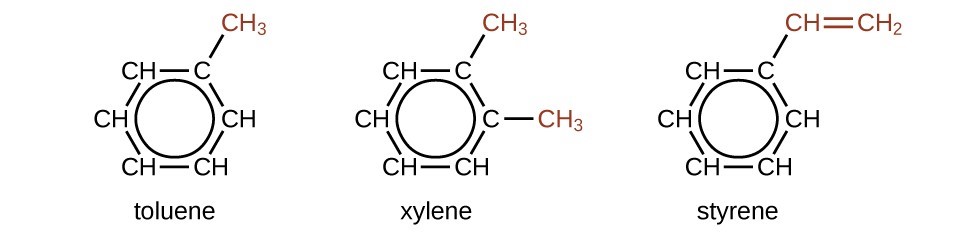
Toluene and xylene are important solvents and raw materials in the chemical industry. Styrene is used to produce the polymer polystyrene.
EXAMPLE
Structure of Aromatic Hydrocarbons
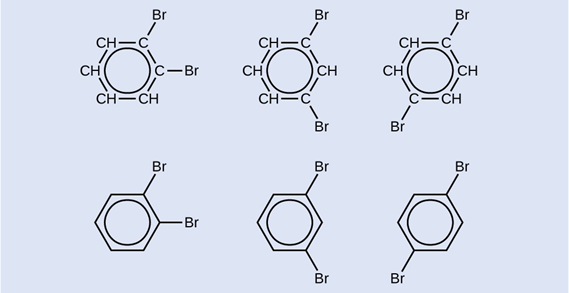
One possible isomer created by a substitution reaction that replaces a hydrogen atom attached to the aromatic ring of toluene with a chlorine atom is shown here. Draw two other possible isomers in which the chlorine atom replaces a different hydrogen atom attached to the aromatic ring: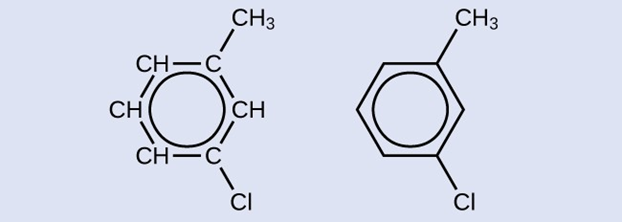
Solution
Since the six-carbon ring with alternating double bonds is necessary for the molecule to be classified as aromatic, appropriate isomers can be produced only by changing the positions of the chloro-substituent relative to the methyl-substituent: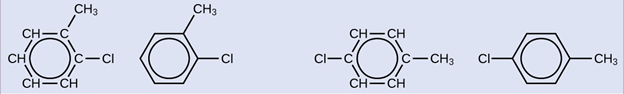
Check Your Learning
Draw three isomers of a six-membered aromatic ring compound substituted with two bromines.
Key Concepts and Summary
Strong, stable bonds between carbon atoms produce complex molecules containing chains, branches, and rings. The chemistry of these compounds is called organic chemistry. Hydrocarbons are organic compounds composed of only carbon and hydrogen. The alkanes are saturated hydrocarbons—that is, hydrocarbons that contain only single bonds. Alkenes contain one or more carbon-carbon double bonds. Alkynes contain one or more carbon-carbon triple bonds. Aromatic hydrocarbons contain ring structures with delocalized π electron systems.
END OF CHAPTER EXERCISES
- Write the chemical formula and Lewis structure of the following, each of which contains five carbon atoms: (a) an alkane (b) an alkene (c) an alkyne
There are several sets of answers; one is:
(a) [latex]\text{C}_5\text{H}_{12}[/latex]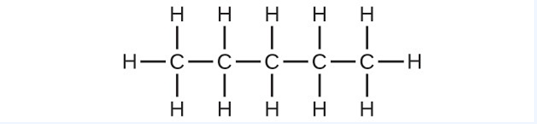
(b) [latex]\text{C}_5\text{H}_{10}[/latex]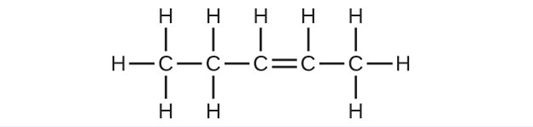 (c) [latex]\text{C}_5\text{H}_8[/latex]
(c) [latex]\text{C}_5\text{H}_8[/latex]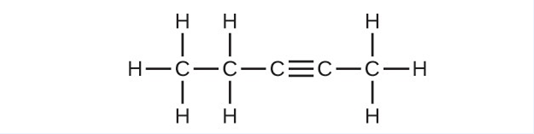
- What is the difference between the hybridization of carbon atoms’ valence orbitals in saturated and unsaturated hydrocarbons?
- On a microscopic level, how does the reaction of bromine with a saturated hydrocarbon differ from its reaction with an unsaturated hydrocarbon? How are they similar?
Both reactions result in bromine being incorporated into the structure of the product. The difference is the way in which that incorporation takes place. In the saturated hydrocarbon, an existing C–H bond is broken, and a bond between the C and the Br can then be formed. In the unsaturated hydrocarbon, the only bond broken in the hydrocarbon is the π bond whose electrons can be used to form a bond to one of the bromine atoms in Br2 (the electrons from the Br–Br bond form the other C–Br bond on the other carbon that was part of the π bond in the starting unsaturated hydrocarbon).
- On a microscopic level, how does the reaction of bromine with an alkene differ from its reaction with an alkyne? How are they similar?
- Explain why unbranched alkenes can form geometric isomers while unbranched alkanes cannot. Does this explanation involve the macroscopic domain or the microscopic domain?
Unbranched alkanes have free rotation about the C–C bonds, yielding all orientations of the substituents about these bonds equivalent, interchangeable by rotation. In the unbranched alkenes, the inability to rotate about the C=C bond results in fixed (unchanging) substituent orientations, thus permitting different isomers. Since these concepts pertain to phenomena at the molecular level, this explanation involves the microscopic domain.
- Explain why these two molecules are not isomers:
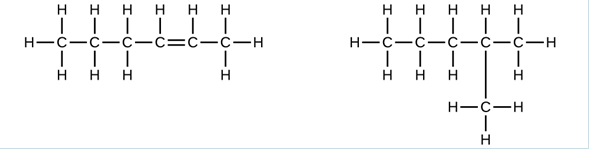
- Explain why these two molecules are not isomers:
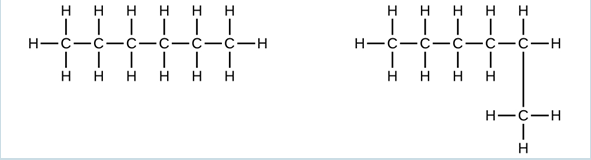 They are the same compound because each is a saturated hydrocarbon containing an unbranched chain of six carbon atoms.
They are the same compound because each is a saturated hydrocarbon containing an unbranched chain of six carbon atoms. - How does the carbon-atom hybridization change when polyethylene is prepared from ethylene?
- Write the Lewis structure and molecular formula for each of the following hydrocarbons:
(a) hexane
(b) 3-methylpentane
(c) cis-3-hexene
(d) 4-methyl-1-pentene
(e) 3-hexyne
(f) 4-methyl-2-pentyne(a) [latex]\text{C}_6\text{H}_{14}[/latex]
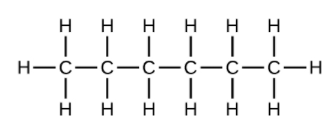
(b) [latex]\text{C}_6\text{H}_{14}[/latex]
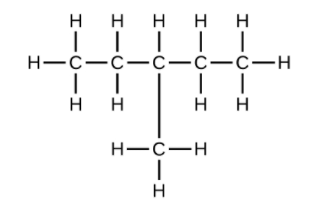
(c) [latex]\text{C}_6\text{H}_{12}[/latex]
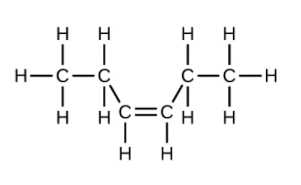
(d) [latex]\text{C}_6\text{H}_12[/latex]
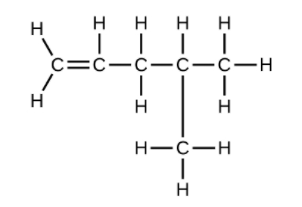
(e) [latex]\text{C}_6\text{H}_{10}[/latex]
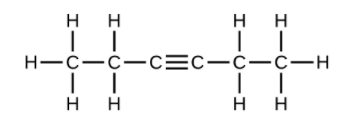
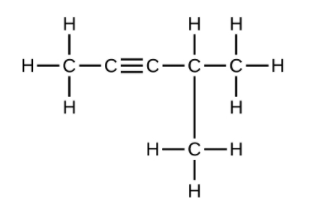
(f) [latex]\text{C}_6\text{H}_{10}[/latex]
- Write the chemical formula, condensed formula, and Lewis structure for each of the following hydrocarbons:
(a) heptane
(b) 3-methylhexane
(c) trans-3-heptene
(d) 4-methyl-1-hexene
(e) 2-heptyne
(f) 3,4-dimethyl-1-pentyne - Give the complete IUPAC name for each of the following compounds:
(a) [latex]\text{CH}_3\text{CH}_2\text{CBr}_2\text{CH}_3[/latex](b) [latex]\text{(CH}_3)_3\text{CCl}[/latex](c)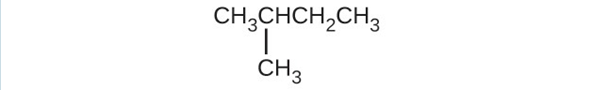
(d) [latex]\text{CH}_3\text{CH}_2\text{C} \equiv \text{CH}[/latex](e)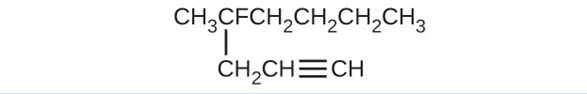
(f)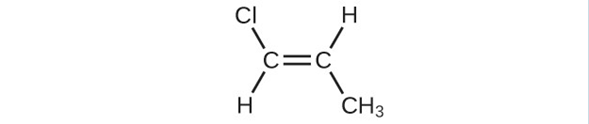
(g) [latex]\text{(CH}_3)_2\text{CHCH}_2\text{CH = CH}_2[/latex](a) 2,2-dibromobutane; (b) 2-chloro-2-methylpropane; (c) 2-methylbutane; (d) 1-butyne; (e) 4-fluoro-4-methyl-1-octyne; (f) trans-1-chloropropene; (g) 5-methyl-1-pentene - Give the complete IUPAC name for each of the following compounds:(a) [latex]\text{(CH}_3)_2\text{CHF}[/latex](b) [latex]\text{CH}_3\text{CHClCHClCH}_3[/latex](c)
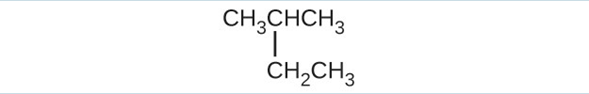
(d) [latex]\text{CH}_3\text{CH}_2\text{CH = CHCH}_3[/latex](e)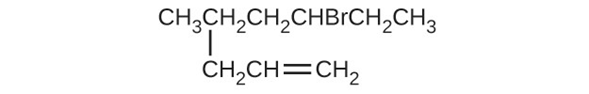
(f) [latex]\text{(CH}_3)_3\text{CCH}_2\text{C} \equiv \text{CH}[/latex] - Butane is used as a fuel in disposable lighters. Write the Lewis structure for each isomer of butane.
- Write Lewis structures and name the five structural isomers of hexane.
- Write Lewis structures for the cis–trans isomers of [latex]\text{CH}_3\text{CH = CHCl}[/latex].
- Write structures for the three isomers of the aromatic hydrocarbon xylene, [latex]\text{C}_6\text{H}_4\text{(CH}_3)_2[/latex].
- Isooctane is the common name of the isomer of [latex]\text{C}_8\text{H}_{18}[/latex] used as the standard of 100 for the gasoline octane rating:
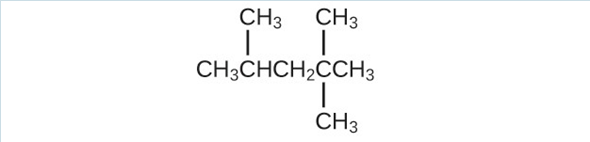
(a) What is the IUPAC name for the compound?
(b) Name the other isomers that contain a five-carbon chain with three methyl substituents.(a) 2,2,4-trimethylpentane; (b) 2,2,3-trimethylpentane, 2,3,4-trimethylpentane, and 2,3,3-trimethylpentane: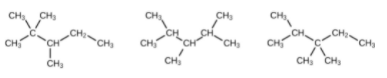
- Write Lewis structures and IUPAC names for the alkyne isomers of [latex]\text{C}_4\text{H}_6[/latex].
- Write Lewis structures and IUPAC names for all isomers of [latex]\text{C}_4\text{H}_9\text{Cl}[/latex].
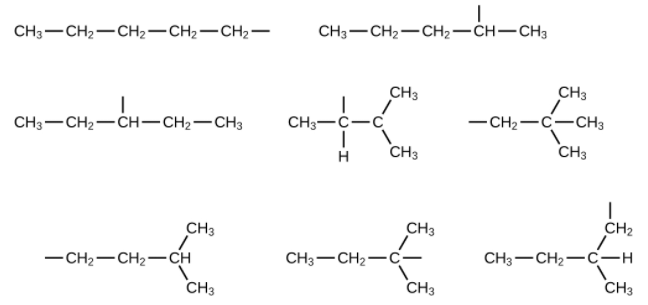
- Name and write the structures of all isomers of the propyl and butyl alkyl groups.
- Write the structures for all the isomers of the [latex]\text{–C}_5\text{H}_{11}[/latex] alkyl group.
In the following, the carbon backbone and the appropriate number of hydrogen atoms are shown in condensed form:
- Write Lewis structures and describe the molecular geometry at each carbon atom in the following compounds: (a) cis-3-hexene (b) cis-1-chloro-2-bromoethene (c) 2-pentyne (d) trans–6-ethyl-7-methyl-2-octene
- Benzene is one of the compounds used as an octane enhancer in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene: [latex]\text{3C}_2\text{H}_2 \rightarrow \text{C}_6\text{H}_6[/latex] Draw Lewis structures for these compounds, with resonance structures as appropriate, and determine the hybridization of the carbon atoms in each.
 In acetylene, the bonding uses sp hybrids on carbon atoms and s orbitals on hydrogen atoms. In benzene, the carbon atoms are sp2 hybridized.
In acetylene, the bonding uses sp hybrids on carbon atoms and s orbitals on hydrogen atoms. In benzene, the carbon atoms are sp2 hybridized. - Teflon is prepared by the polymerization of tetrafluoroethylene. Write the equation that describes the polymerization using Lewis symbols.
- Write two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures. (a) 1 mol of 1-butyne reacts with 2 mol of iodine. (b) Pentane is burned in air.
(a) [latex]\text{CH} \equiv \text{CCH}_2\text{CH}_3 + \text{2I}_2 \rightarrow \text{CHI}_2\text{CI}_2\text{CH}_2\text{CH}_3[/latex]
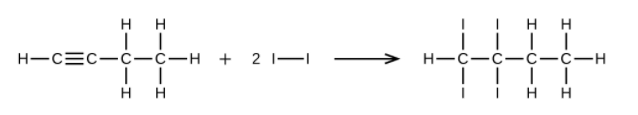
(b) [latex]\text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2\text{CH}_3 + \text{8O}_2\rightarrow \text{5CO}_2 + \text{6H}_2\text{O}[/latex]
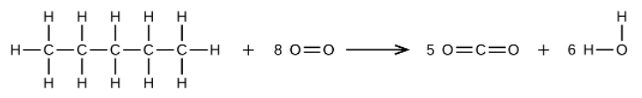
- Write two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures. (a) 2-butene reacts with chlorine. (b) benzene burns in air.
- What mass of 2-bromopropane could be prepared from 25.5 g of propene? Assume a 100% yield of product.
65.2 g
- Acetylene is a very weak acid; however, it will react with moist silver(I) oxide and form water and a compound composed of silver and carbon. Addition of a solution of [latex]\text{HCl}[/latex] to a 0.2352-g sample of the compound of silver and carbon produced acetylene and 0.2822 g of [latex]\text{AgCl}[/latex]. (a) What is the empirical formula of the compound of silver and carbon? (b) The production of acetylene on addition of [latex]\text{HCl}[/latex] to the compound of silver and carbon suggests that the carbon is present as the acetylide ion, [latex]\text{C}_2 ^{2-[/latex]. Write the formula of the compound showing the acetylide ion.
- Ethylene can be produced by the pyrolysis of ethane: [latex]\text{C}_2\text{H}_6 \rightarrow \text{C}_2\text{H}_4 + \text{H}_2[/latex] How many kilograms of ethylene is produced by the pyrolysis of 1.000 × 103 kg of ethane, assuming a 100.0% yield?
9.328 × 102 kg
Footnotes
- 1 This is the Beilstein database, now available through the Reaxys site (www.elsevier.com/online-tools/reaxys).
- 2 Peplow, Mark. “Organic Synthesis: The Robo-Chemist,” Nature 512 (2014): 20–2.
- 3 Physical properties for C4H10 and heavier molecules are those of the normal isomer, n-butane, n-pentane, etc.
- 4 STP indicates a temperature of 0 °C and a pressure of 1 atm.
Glossary
- addition reaction
- reaction in which a double carbon-carbon bond forms a single carbon-carbon bond by the addition of a reactant. Typical reaction for an alkene.
- alkane
- molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
- alkene
- molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
- alkyl group
- substituent, consisting of an alkane missing one hydrogen atom, attached to a larger structure
- alkyne
- molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
- aromatic hydrocarbon
- cyclic molecule consisting of carbon and hydrogen with delocalized alternating carbon-carbon single and double bonds, resulting in enhanced stability
- functional group
- part of an organic molecule that imparts a specific chemical reactivity to the molecule
- organic compound
- natural or synthetic compound that contains carbon
- saturated hydrocarbon
- molecule containing carbon and hydrogen that has only single bonds between carbon atoms
- skeletal structure
- shorthand method of drawing organic molecules in which carbon atoms are represented by the ends of lines and bends in between lines, and hydrogen atoms attached to the carbon atoms are not shown (but are understood to be present by the context of the structure)
- substituents
- branch or functional group that replaces hydrogen atoms in a larger hydrocarbon chain
- substitution reaction
- reaction in which one atom replaces another in a molecule
This chapter is an adaptation of the chapter “Hydrocarbons” in Chemistry: Atoms First 2e by OpenStax and is licensed under a CC BY 4.0 license.
Access for free at https://openstax.org/books/chemistry-atoms-first-2e/pages/1-introduction
natural or synthetic compound that contains carbon
molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
molecule containing carbon and hydrogen that has only single bonds between carbon atoms
shorthand method of drawing organic molecules in which carbon atoms are represented by the ends of lines and bends in between lines, and hydrogen atoms attached to the carbon atoms are not shown (but are understood to be present by the context of the structure)
branch or functional group that replaces hydrogen atoms in a larger hydrocarbon chain
substituent, consisting of an alkane missing one hydrogen atom, attached to a larger structure
reaction in which one atom replaces another in a molecule
part of an organic molecule that imparts a specific chemical reactivity to the molecule
molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
reaction in which a double carbon-carbon bond forms a single carbon-carbon bond by the addition of a reactant. Typical reaction for an alkene.
molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
cyclic molecule consisting of carbon and hydrogen with delocalized alternating carbon-carbon single and double bonds, resulting in enhanced stability
element in group 15
inner transition metal in the top of the bottom two rows of the periodic table
similar to internal radiation therapy, but chemical rather than radioactive substances are introduced into the body to kill cancer cells
radiation delivered by a machine outside the body
(also, brachytherapy) radiation from a radioactive substance introduced into the body to kill cancer cells
use of high-energy radiation to damage the DNA of cancer cells, which kills them or keeps them from dividing
(also, radioactive label) radioisotope used to track or follow a substance by monitoring its radioactive emissions
(also, noble gas) element in group 18


