51 Intermolecular Forces
LumenLearning
Dipole-Dipole Force
Dipole-dipole interactions are intermolecular attractions that result from two permanent dipoles interacting.
LEARNING OBJECTIVES
Explain the cause of a dipole-dipole force.
KEY TAKEAWAYS
Key Points
- Dipole-dipole interactions occur when the partial charges formed within one molecule are attracted to an opposite partial charge in a nearby molecule.
- Polar molecules align so that the positive end of one molecule interacts with the negative end of another molecule.
- Unlike covalent bonds between atoms within a molecule (intramolecular bonding), dipole-dipole interactions create attractions between molecules of a substance (intermolecular attractions).
Key Terms
- hydrogen bond: An intermolecular attraction between a partially positively charged hydrogen in one molecule and a partially negatively charged oxygen, nitrogen, or fluorine in a nearby molecule.
- dipole: In chemistry, a permanent dipole describes the partial charge separation that can occur within a molecule along the bond that forms between two different atoms. Dipoles generally occur between two nonmetals that share electrons as part of their bond. Since each atom has a different affinity for electrons, the ‘push and pull’ of their shared electrons results in one atom maintaining most of the electron density and a partial negative charge, leaving the other atom with a partial positive charge.
- polar: In chemistry, a polar molecule is one that has uneven charge distribution. Factors that contribute to this include intramolecular dipoles and molecular geometry.
Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions ). These forces are weak compared to the intramolecular forces, such as the covalent bonds between atoms in a molecule or ionic bonds between atoms in an ionic compound. For example, the covalent bond present within a hydrogen chloride ([latex]\text{HCl}[/latex]) molecule is much stronger than any attraction/repulsion it has with neighboring molecules.
Types of Attractive Intermolecular Forces
- Dipole-dipole forces: electrostatic interactions of permanent dipoles in molecules; includes hydrogen bonding.
- Ion-dipole forces: electrostatic interaction involving a permanent dipole of one molecule and a fully charged ion.
- Instantaneous dipole-induced dipole forces or London dispersion forces: forces caused by correlated movements of the electrons in interacting molecules, which are the weakest of intermolecular forces and are categorized as van der Waals forces.
Dipole-Dipole Attractions
Dipole–dipole interactions are a type of intermolecular attraction—attractions between two molecules. Dipole-dipole interactions are electrostatic interactions between the permanent dipoles of different molecules. These interactions align the molecules to increase the attraction.
An electric monopole is a single charge, while a dipole is two opposite charges closely spaced to each other. Molecules that contain dipoles are called polar molecules and are very abundant in nature. For example, a water molecule ([latex]\text{H}_2\text{O}[/latex]) has a large permanent electric dipole moment. Its positive and negative charges are not centered at the same point; it behaves like a few equal and opposite charges separated by a small distance. These dipole-dipole attractions give water many of its properties, including its high surface tension.
Uneven Distribution of Electrons
The permanent dipole in water is caused by oxygen ‘s tendency to draw electrons to itself (i.e. oxygen is more electronegative than hydrogen). The 10 electrons of a water molecule are found more regularly near the oxygen atom. As a result, oxygen has a slight negative charge (δ-). The electrons are found less regularly around the hydrogen atoms. As a result, hydrogen has a slight positive charge (δ+).
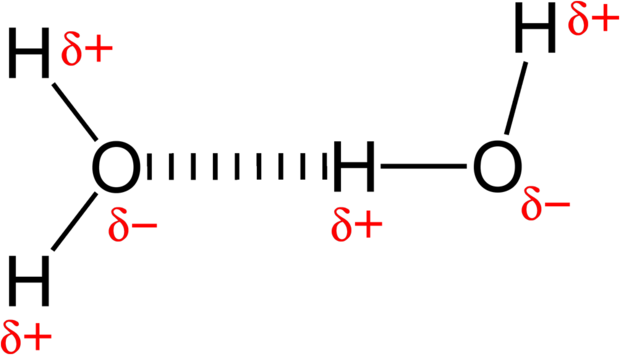
Examples of Dipole-Dipole Interactions
Another example of a dipole–dipole interaction can be seen in hydrogen chloride ([latex]\text{HCl}[/latex]): the relatively positive end of a polar molecule will attract the relatively negative end of another [latex]\text{HCl}[/latex] molecule. The interaction between the two dipoles is an attraction rather than full bond because no electrons are shared between the two molecules.
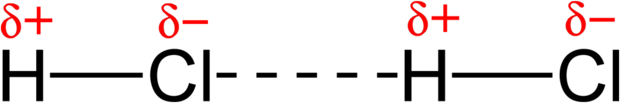
Symmetrical Molecules with No Overall Dipole Moment
Molecules often contain polar bonds because of electronegativity differences but have no overall dipole moment if they are symmetrical. For example, in the molecule tetrachloromethane ([latex]\text{CCl}_4[/latex]), the chlorine atoms are more electronegative than the carbon atoms, and the electrons are drawn toward the chlorine atoms creating dipoles. However, these carbon-chlorine dipoles cancel each other out because the molecular is symmetrical, and [latex]\text{CCl}_4[/latex] has no overall dipole movement.
Hydrogen Bonds
Hydrogen bonds are a type of dipole-dipole interactions that occur between hydrogen and either nitrogen, fluorine, or oxygen. Hydrogen bonds are incredibly important in biology, because hydrogen bonds keep the DNA bases paired together, helping DNA maintain its unique structure.
Dipole Forces – YouTube: In this video, Paul Andersen describes the intermolecular forces associated with dipoles. A dipole is a molecule that has split charge. Dipoles may form associations with other dipoles, induced dipoles or ions. An important type of dipole-dipole forces are hydrogen bonds.
Hydrogen Bonding
A hydrogen bond is a strong intermolecular force created by the relative positivity of hydrogen atoms.
LEARNING OBJECTIVES
Describe the properties of hydrogen bonding.
KEY TAKEAWAYS
Key Points
- Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to a highly electronegative atom approaches a nearby electronegative atom.
- Greater electronegativity of the hydrogen bond acceptor will lead to an increase in hydrogen bond strength.
- The hydrogen bond is one of the strongest intermolecular attractions, but is weaker than a covalent or an ionic bond.
- Hydrogen bonds are responsible for holding together DNA, proteins, and other macromolecules.
Key Terms
- electronegativity: The tendency of an atom or molecule to draw electrons towards itself, form dipoles, and thus form bonds.
- hydrogen bond: The attraction between a partially positively charged hydrogen atom attached to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and another nearby electronegative atom.
- intermolecular: A type of interaction between two different molecules.
Forming a Hydrogen Bond
A hydrogen bond is the electromagnetic attraction created between a partially positively charged hydrogen atom attached to a highly electronegative atom and another nearby electronegative atom. A hydrogen bond is a type of dipole-dipole interaction; it is not a true chemical bond. These attractions can occur between molecules (intermolecularly) or within different parts of a single molecule (intramolecularly).
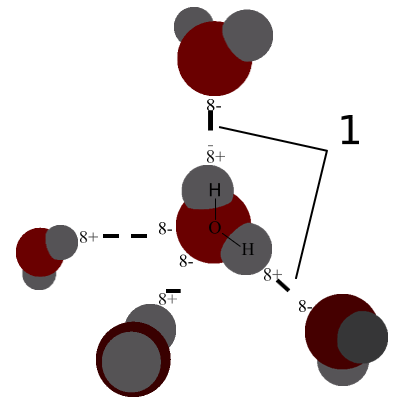
Hydrogen Bond Donor
A hydrogen atom attached to a relatively electronegative atom is a hydrogen bond donor. This electronegative atom is usually fluorine, oxygen, or nitrogen. The electronegative atom attracts the electron cloud from around the hydrogen nucleus and, by decentralizing the cloud, leaves the hydrogen atom with a positive partial charge. Because of the small size of hydrogen relative to other atoms and molecules, the resulting charge, though only partial, is stronger. In the molecule ethanol, there is one hydrogen atom bonded to an oxygen atom, which is very electronegative. This hydrogen atom is a hydrogen bond donor.
Hydrogen Bond Acceptor
A hydrogen bond results when this strong partial positive charge attracts a lone pair of electrons on another atom, which becomes the hydrogen bond acceptor. An electronegative atom such as fluorine, oxygen, or nitrogen is a hydrogen bond acceptor, regardless of whether it is bonded to a hydrogen atom or not. Greater electronegativity of the hydrogen bond acceptor will create a stronger hydrogen bond.
Applications for Hydrogen Bonds
Hydrogen bonds occur in inorganic molecules, such as water, and organic molecules, such as DNA and proteins. The two complementary strands of DNA are held together by hydrogen bonds between complementary nucleotides (A&T, C&G). Hydrogen bonding in water contributes to its unique properties, including its high boiling point (100 °C) and surface tension.

In biology, intramolecular hydrogen bonding is partly responsible for the secondary, tertiary, and quaternary structures of proteins and nucleic acids. The hydrogen bonds help the proteins and nucleic acids form and maintain specific shapes.
Ion-Dipole Force
The ion-dipole force is an intermolecular attraction between an ion and a polar molecule.
LEARNING OBJECTIVES
Define ion-dipole force.
KEY TAKEAWAYS
Key Points
- An ion – dipole interaction occurs between a fully charged ion and a partially charged dipole.
- The strength of the ion-dipole force is proportionate to ion charge.
- An ion-induced dipole interaction occurs between a fully charged ion and a temporarily charged dipole. The temporary dipole is induced by the presence of the ion.
Key Terms
- ion: An atom or group of atoms bearing an electrical charge, such as sodium and chlorine in table salt.
- ion-dipole forces: An electrostatic interaction involving a permanent dipole in one molecule and an ion.
- ion-induced dipole force: An electrostatic interaction involving a temporary dipole in one molecule and a permanently charged ion.
Ion-Dipole Force
Ion-dipole and ion-induced dipole forces operate much like dipole-dipole and induced dipole-dipole interactions. However, ion-dipole forces involve ions instead of solely polar molecules. Ion-dipole forces are stronger than dipole interactions because the charge of any ion is much greater than the charge of a dipole; the strength of the ion-dipole force is proportionate to ion charge. Ion-dipole bonding is also stronger than hydrogen bonding. An ion-dipole force consists of an ion and a polar molecule aligning so that the positive and negative charges are next to one another, allowing for maximum attraction.
Ion-dipole forces are generated between polar water molecules and a sodium ion. The oxygen atom in the water molecule has a slight negative charge and is attracted to the positive sodium ion. These intermolecular ion-dipole forces are much weaker than covalent or ionic bonds.
Ion-Induced Dipole Force
An ion-induced dipole force occurs when an ion interacts with a non-polar molecule. Like a dipole-induced dipole force, the charge of the ion causes a distortion of the electron cloud in the non-polar molecule, causing a temporary partial charge. The temporary partially charged dipole and the ion are attracted to each other and form a fleeting interaction.
Dispersion Force
Dispersion forces are weak intermolecular forces caused by temporary dipoles.
LEARNING OBJECTIVES
Discuss the characteristics of dispersion forces.
KEY TAKEAWAYS
Key Points
- London dispersion forces are weak intermolecular forces and are considered van der Waals forces.
- Temporary dipoles can occur in non-polar molecules when the electrons that constantly orbit the nucleus occupy a similar location by chance.
- Temporary dipoles can induce a dipole in neighboring molecules, initiating an attraction called a London dispersion force.
Key Terms
- London dispersion forces: A weak intermolecular interaction arising from induced instantaneous dipoles in molecules; part of the Van der Waals forces.
- dipole: Any molecule that has both slight positive and negative charges on either end.
- Van der Waals forces: The sum of the attractive or repulsive forces between molecules (or between parts of the same molecule) other than those due to covalent bonds, or the electrostatic interaction of ions with one another, with neutral molecules, or with charged molecules.
Temporary Dipoles
Temporary dipoles are created when electrons, which are in constant movement around the nucleus, spontaneously come into close proximity. This uneven distribution of electrons can make one side of the atom more negatively charged than the other, thus creating a temporary dipole, even on a non-polar molecule. The more electrons there are in an atom, the further away the shells are from the nucleus; thus, the electrons can become lopsided more easily, and these forces are stronger and more frequent. These intermolecular forces are also sometimes called “induced dipole-induced dipole” or “momentary dipole” forces.
London Dispersion Forces
Although charges are usually distributed evenly between atoms in non-polar molecules, spontaneous dipoles can still occur. When this occurs, non-polar molecules form weak attractions with other non-polar molecules. These London dispersion forces are often found in the halogens (e.g., [latex]\text{F}_2[/latex] and [latex]\text{I}_2[/latex]), the noble gases (e.g., [latex]\text{Ne}[/latex] and [latex]\text{Ar}[/latex]), and in other non-polar molecules, such as carbon dioxide and methane. London dispersion forces are part of the van der Waals forces, or weak intermolecular attractions.
Interactive: Charged and Neural Atoms: There are two kinds of attractive forces shown in this model: Coulomb forces (the attraction between ions) and Van der Waals forces (an additional attractive force between all atoms). What kinds of patterns tend to form with charged and neutral atoms? How does changing the Van der Waals attraction or charging the atoms affect the melting and boiling point of the substance?
Interactive: Comparing Dipole-Dipole to London Dispersion: Investigate the difference in the attractive force between polar and non-polar molecules.
Van der Waals forces help explain how nitrogen can be liquefied. Nitrogen gas ([latex]\text{N}_2[/latex]) is diatomic and non-polar because both nitrogen atoms have the same degree of electronegativity. If there are no dipoles, what would make the nitrogen atoms stick together to form a liquid? London dispersion forces allow otherwise non-polar molecules to have attractive forces. However, they are by far the weakest forces that hold molecules together.

LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Electrodynamics/Dipoles and Multipoles. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/Electrodynamics/Dipoles_and_Multipoles. License: CC BY-SA: Attribution-ShareAlike
- Intermolecular force. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23Dipole-dipole_interactions. License: CC BY-SA: Attribution-ShareAlike
- potential energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/potential%20energy. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- van der Waals forces. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Van_der_Waals_force. License: CC BY-SA: Attribution-ShareAlike
- Dipole Forces – YouTube. Located at: http://www.youtube.com/watch?v=cERb1d6J4-M. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Hydrogen-bonding-in-water-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23mediaviewer/File:Hydrogen-bonding-in-water-2D.png. License: CC BY-SA: Attribution-ShareAlike
- Dipole-dipole-interaction-in-HCl-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Dipole-dipole-interaction-in-HCl-2D.png. License: CC BY-SA: Attribution-ShareAlike
- Hydrogen bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Hydrogen_bond. License: CC BY-SA: Attribution-ShareAlike
- electronegativity. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/electronegativity. License: CC BY-SA: Attribution-ShareAlike
- intermolecular. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/intermolecular. License: CC BY-SA: Attribution-ShareAlike
- Dipole Forces – YouTube. Located at: http://www.youtube.com/watch?v=cERb1d6J4-M. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Hydrogen-bonding-in-water-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23mediaviewer/File:Hydrogen-bonding-in-water-2D.png. License: CC BY-SA: Attribution-ShareAlike
- Dipole-dipole-interaction-in-HCl-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Dipole-dipole-interaction-in-HCl-2D.png. License: CC BY-SA: Attribution-ShareAlike
- File:3D model hydrogen bonds in water.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:3D_model_hydrogen_bonds_in_water.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Water_drops_on_green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- Ion-Dipole Force. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ion-Dipole_Force%23Ion-dipole_and_ion-induced_dipole_forces. License: CC BY-SA: Attribution-ShareAlike
- ion. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ion. License: CC BY-SA: Attribution-ShareAlike
- Intermolecular force. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23Dipole-dipole_interactions. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: http://www.boundless.com//chemistry/definition/ion-dipole-forces. License: CC BY-SA: Attribution-ShareAlike
- Dipole Forces – YouTube. Located at: http://www.youtube.com/watch?v=cERb1d6J4-M. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Hydrogen-bonding-in-water-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23mediaviewer/File:Hydrogen-bonding-in-water-2D.png. License: CC BY-SA: Attribution-ShareAlike
- Dipole-dipole-interaction-in-HCl-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Dipole-dipole-interaction-in-HCl-2D.png. License: CC BY-SA: Attribution-ShareAlike
- File:3D model hydrogen bonds in water.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:3D_model_hydrogen_bonds_in_water.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Water_drops_on_green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- General Chemistry/Intermolecular bonds. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/General_Chemistry/Intermolecular_bonds. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- nonpolar. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nonpolar. License: CC BY-SA: Attribution-ShareAlike
- polar. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/polar. License: CC BY-SA: Attribution-ShareAlike
- London dispersion force. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/London_dispersion_force. License: CC BY-SA: Attribution-ShareAlike
- Dipole Forces – YouTube. Located at: http://www.youtube.com/watch?v=cERb1d6J4-M. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Hydrogen-bonding-in-water-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Intermolecular_force%23mediaviewer/File:Hydrogen-bonding-in-water-2D.png. License: CC BY-SA: Attribution-ShareAlike
- Dipole-dipole-interaction-in-HCl-2D. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Dipole-dipole-interaction-in-HCl-2D.png. License: CC BY-SA: Attribution-ShareAlike
- File:3D model hydrogen bonds in water.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:3D_model_hydrogen_bonds_in_water.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Water drops on green leaf. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Water_drops_on_green_leaf.jpg. License: CC BY-SA: Attribution-ShareAlike
- Liquidnitrogen. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Liquidnitrogen.jpg. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter “Intermolecular Forces” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
unit of concentration, defined as the number of moles of solute dissolved in 1 liter of solution
Chemical structures from Dalton’s A New System of Chemical Philosophy.

