81 Synthetic Organic Polymers
LumenLearning
Types of Synthetic Organic Polymers
Synthetic organic polymers are human-made polymers with various main chain and side chain compositions.
LEARNING OBJECTIVES
Describe how the chemical structure of a polymer relates to its physical properties.
KEY TAKEAWAYS
Key Points
- Synthetic polymers are human-made polymers. They can be classified into four main categories: thermoplastics, thermosets, elastomers, and synthetic fibers. They are commonly found in a variety of consumer products.
- Various main chains and side chains are used to make different synthetic organic polymers. The backbones of common synthetic polymers are made of carbon-carbon bonds, whereas heterochain polymers have other elements (e.g. oxygen, sulfur, nitrogen) inserted along the backbone.
- The seven most common types of synthetic organic polymers are: low density polyethylene (LDPE), high density polyethylene (HDPE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), nylon, Teflon, and thermoplastic polyurethane (TPU).
Key Terms
- thermoplastic: a polymer that becomes pliable or moldable above a specific temperature and returns to a solid state upon cooling
- synthetic polymers: human-made polymers
Synthetic polymers are human-made polymers. From the utility point of view, they can be classified into four main categories: thermoplastics, thermosets, elastomers, and synthetic fibers. Thermoplastics are a type of polymer that become moldable and malleable past a certain temperature, and they solidify upon cooling. Similarly, thermosets similarly become hard and cannot change shape once they have set; for this reason, they are often used in adhesives. An elastomer—a term used interchangeably with rubber—is a flexible polymer. Synthetic fibers are created by improving upon natural plant and animal fibers and make up a large category of polymers.
Poly acrylates are the backbones of common synthetic polymers such as polythene and polystyrene. They are made up of carbon-carbon bonds, whereas hetero chain polymers such as polyamides, polyesters, polyurethanes, polysulfides, and polycarbonates have other elements (e.g. oxygen, sulfur, nitrogen) inserted along the backbone. Coordination polymers may contain a range of metals in the backbone, with non-covalent bonding present. A wide variety of synthetic polymers are also available with variations in their main chains and side chains.
Synthetic Polymers in Everyday Use
Some familiar household synthetic polymers include nylons in textiles and fabrics, Teflon in non-stick pans, and polyvinyl chloride in pipes. The common PET bottles are made of a synthetic polymer, polyethylene terephthalate. The plastic kits and covers are mostly made of synthetic polymers like polythene, and tires are manufactured from Buna rubbers. Due to the environmental issues created by these synthetic polymers, which are often non-biodegradable and synthesized from petroleum, alternatives like bioplastics are also being considered; these bioplastics are often more expensive than synthetic polymers, however.
Many polymers are made entirely from hydrocarbons. This makes them hydrophobic, meaning they do not readily absorb water; this is a useful trait, as the alternative—imagine a water bottle that becomes soggy when filled with water, for instance—might be disastrous.
Types of Synthetic Polymers
Low Density Polyethylene
Low Density Polyethylene (LDPE) polymers are among the most common types of synthetic organic polymers, which are often found in households. LDPE is a thermoplastic made from the monomer ethylene. One of the first polymers to be created, it was produced in 1933 by Imperial Chemical Industries using a high pressure process via free radical polymerization. It is manufactured the way method today. LDPE is commonly recycled, with the number 4 as its recycling symbol. Despite competition from more modern polymers, LDPE continues to be an important plastic grade.
High Density Polyethylene
High Density Polyethylene (HDPE) or polyethylene high-density (PEHD) is a polyethylene thermoplastic made from petroleum. It takes 1.75 kilograms of petroleum (in terms of energy and raw materials) to make one kilogram of HDPE. HDPE is commonly recycled, with the number 2 as its recycling symbol.
Polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications, including packaging and labeling, textiles, stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes. An additional polymer made from the monomer propylene, it is rugged and unusually resistant to many chemical solvents, bases, and acids.
Polyvinyl Chloride
Polyvinyl Chloride (PVC) is the third-most widely produced plastic, after polyethylene and polypropylene. PVC is used in construction because it is cheaper and stronger than more traditional alternatives such as copper or ductile iron. It can be made softer and more flexible by adding plasticizers, the most popular of which are phthalates. In this form, PVC is used in clothing and upholstery, electrical cable insulation, inflatable products, and many applications in which it replaces rubber.
Polystyrene
Polystyrene (PS) is an aromatic polymer made from the monomer styrene, a liquid petrochemical. One of the most popular plastics, PS is a colorless solid that is used, for example, in disposable cutlery, plastic models, CD and DVD cases, and smoke detector housings. Products made from foamed polystyrene include packing materials, insulation, and foam drink cups. Its very slow biodegradation is a focus of controversy, and it can often be found littered outdoors, particularly along shores and waterways.
Nylon
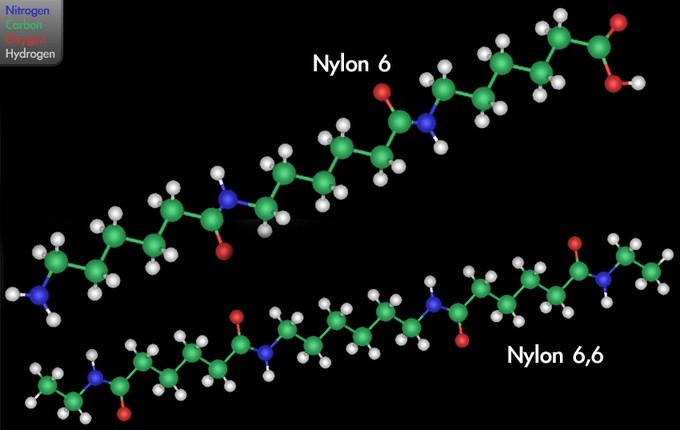
Nylon, a family of synthetic polymers known generically as polyamides, was first produced on February 28, 1935 by Wallace Carothers at DuPont’s research facility. Nylon is one of the most commonly-used polymers. The amide backbone present in nylon causes it to be more hydrophilic than the polymers discussed above. Notice that your nylon clothing will absorb water, for instance; this is because nylon can engage in hydrogen bonding with water, unlike the purely hydrocarbon polymers that make up most plastics.
Teflon

Teflon (Polytetrafluoroethylene or PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications. PTFE is a solid, high-molecular-weight compound consisting entirely of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances can interact with PTFE. PTFE is used as a non-stick coating for pans and other cookware because it has very low friction with other compounds. It is very non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. Where used as a lubricant, PTFE reduces friction, wear, and energy consumption of machinery. Although the widespread belief that Teflon is the result of NASA space projects is not true, it has been used by NASA.
Thermoplastic Polyurethane
Thermoplastic polyurethane (TPU) is any of a class of polyurethane plastic. It has many useful properties, including elasticity, transparency, and resistance to oil, grease, and abrasion. Most of these properties are resultant of the fact that TPU is hydrophilic and can react with water. Technically, TPU is a thermoplastic elastomer consisting of linear segmented block copolymers made of hard and soft segments.
Addition Reactions
In addition reaction, two molecules combine to create one bigger molecule.
LEARNING OBJECTIVES
Define an addition reaction.
KEY TAKEAWAYS
Key Points
- An addition reaction is an organic reaction in which two or more molecules recombine to form a larger molecule. The reaction only occurs between chemical compounds that have multiple bonds. An elimination reaction is the opposite of an addition reaction.
- Polar addition reactions are electrophilic and nucleophilic additions: in electrophilic additions, electrons from the double bond “attack” another compound to form bonds; in nucleophilic additions, a pair of electrons from the new compound “attack” the double bond to form new single bonds.
- Non-polar addition reactions include free radical additions. Free radicals attack the double bonds so that new single bonds form and the radical will no longer have an unpaired electron.
- In an addition-elimination reaction, elimination reaction takes place after addition reaction.
Key Terms
- addition reaction: an organic reaction in which two or more molecules combine to form a larger molecule
- radical: a very reactive substance with an unpaired electron
- electrophilic addition: a reaction in which a compound is added to an alkene by breaking the double bond to create new single bonds to the additional compound
In organic chemistry, an addition reaction is, in its simplest terms, an organic reaction in which two or more molecules combine to form a larger molecule. Addition reactions are limited to chemical compounds that have multiple bonds, such as molecules with carbon-carbon double bonds (alkenes) or with triple bonds (alkynes). Molecules containing carbon based double bonds, like carbonyl ([latex]\text{C=O}[/latex]) groups or imine ([latex]\text{C=N}[/latex]) groups, can also undergo addition. An addition reaction is the opposite of an elimination reaction. For instance, an alkene’s hydration reaction adds water to an alkene, and an alcohol ‘s dehydration removes water from the alkene; these two reactions are opposites and are considered addition-elimination pairs.
There are two main types of polar addition reactions: electrophilic addition and nucleophilic addition. Two non-polar addition reactions also exist: free radical addition and cycloadditions. In the related addition-elimination reaction, an addition reaction is followed by an elimination reaction; in most reactions, this involves addition to carbonyl compounds in nucleophilic acyl substitution. The hydrolysis of nitriles to carboxylic acids is also a form of addition-elimination.
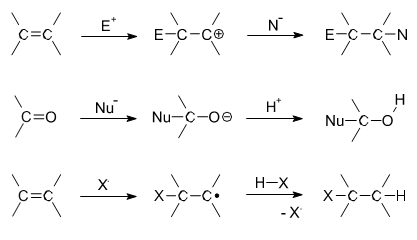
Electrophilic Addition
Most addition reactions to alkenes follow the mechanism of electrophilic addition. An example is the Prins reaction, where the electrophile is a carbonyl group. In halogenation, adding elementary bromine or chlorine to alkenes yields dibromo- and dichloro- alkanes, respectively.
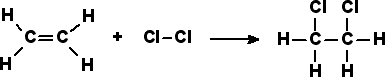
Nucleophilic Addition Reactions
In nucleophilic addition reactions, the nucleophile donates an electron pair to the electrophile (one of the atoms in the double bond). In hydrohalogenation, the nucleophile is the halogen. The halogen donates an electron pair to one of the carbons participating in a double bond. The hydrogen is then added to the other carbon that was originally double bonded. Addition of hydrohalic acids like [latex]\text{HCl}[/latex] or [latex]\text{HBr}[/latex] to alkenes yields the corresponding haloalkanes. An example of this type of reaction is:
[latex]\text{CH}_3\text{CH} = \text{CH}_2 + \text{HBr} \rightarrow \text{CH}_3 - \text{CHBr} - \text{CH}_3[/latex]
Free Radical Additions of Halogens to Alkanes
In free radical additions of halogens to alkanes (or alkenes), a radical halogen can attack an alkane to produce another radical, in this case a radical version of the alkane. The radical alkane can attack another compound, producing another radical that can continue on to attack another compound.
Polymerization of alkenes is an economically important reaction that yields polymers of high industrial value, such as the plastics polyethylene and polypropylene. Polymerization can either proceed via a free-radical or an ionic mechanism.
Condensation Reactions
Condensation is a chemical reaction in which one molecule is formed and one small molecule (often water) is lost.
LEARNING OBJECTIVES
Recognize the chemical principles of condensation reactions as they relate to polymerization.
KEY TAKEAWAYS
Key Points
- During condensation reaction, two molecules combine to form a single molecule with the loss of a small molecule; in dehydration reaction, this lost molecule is water.
- Intermolecular condensation occurs between two separate molecules, while intramolecular condensation is the union between atoms or groups of the same molecule, often leading to ring formation.
- Condensation reactions are used in condensation polymerization, when a series of condensation steps form long chains; this reaction may be either a homopolymerization of a single monomer or a copolymerization of two co-monomers; many biological transformations are condensation reactions.
Key Terms
- condensation reaction: any reaction in which two molecules react with the resulting loss of a water molecule (or other small molecule); the formal reverse of hydrolysis
- condensation polymerization: a polymerization mechanism in which monomers react to form dimers first, then trimers, longer oligomers, and eventually long chain polymers
- dehydration reaction: an elimination (condensation) reaction in which the small molecule that is removed is water
In a condensation reaction, two molecules or parts thereof combine, releasing a small molecule. When this small molecule is water, it is known as a dehydration reaction. Other possible lost molecules include hydrogen chloride, methanol, and acetic acid.
When two separate molecules react, their condensation is termed intermolecular. A simple example is the condensation of two amino acids to form a peptide. This reaction example is the reverse of hydrolysis, which splits a chemical entity into two parts through action from the polar water molecule, which itself splits into hydroxide and hydrogen ions.
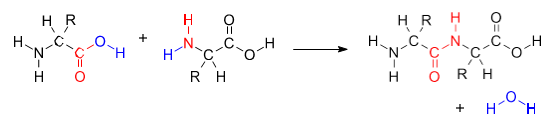
When a condensation is performed between different parts of the same molecule, the reaction is termed intramolecular condensation; in many cases, this leads to ring formation. An example is the Dieckmann condensation, in which the two ester groups of a single diester molecule react with each other to lose a small alcohol molecule and form a β-ketoester product.
Many condensation reactions follow a nucleophilic acyl substitution or an aldol condensation reaction mechanism (see previous concept for more information). Other condensations, such as the acyloin condensation, are triggered by radical conditions.
Condensation Polymerization Reactions
In one type of polymerization reaction, a series of condensation steps takes place whereby monomers or monomer chains add to each other to form longer chains. This is termed “condensation polymerization,” or “step-growth polymerization,” and occurs in such processes as the synthesis of polyesters or nylons. Nylon is a silky material used to make clothes made of repeating units linked by amide bonds, and is frequently referred to as polyamide. This reaction may be either a homopolymerization of a single monomer [latex]\text{A-B}[/latex] with two different end groups that condense, or a copolymerization of two co-monomers [latex]\text{A-A}[/latex] and [latex]\text{B-B}[/latex]. Small molecules are usually liberated in these condensation steps, unlike polyaddition reactions.
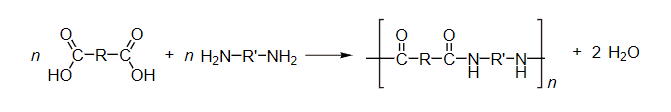
Condensation polymers often require heat, form slower than do addition polymers, and are lower in molecular weight. This type of reaction is used as a basis for making many important polymers, such as nylon, polyester, and various epoxies. It is also the basis for the laboratory formation of silicates and polyphosphates. Many biological transformations, such as polypeptide synthesis, polyketide synthesis, terpene syntheses, phosphorylation, and glycosylations are condensations.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Polystyrene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Polystyrene. License: CC BY-SA: Attribution-ShareAlike
- Polypropylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Polypropylene. License: CC BY-SA: Attribution-ShareAlike
- Nylon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Nylon. License: CC BY-SA: Attribution-ShareAlike
- Teflon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Teflon. License: CC BY-SA: Attribution-ShareAlike
- Polyvinyl Chloride. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Polyvinyl_Chloride. License: CC BY-SA: Attribution-ShareAlike
- High Density Polyethylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/High_Density_Polyethylene. License: CC BY-SA: Attribution-ShareAlike
- Low Density Polyethylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Low_Density_Polyethylene. License: CC BY-SA: Attribution-ShareAlike
- Thermoplastic polyurethanes. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Thermoplastic_polyurethanes. License: CC BY-SA: Attribution-ShareAlike
- Polymer. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Polymer%23Polymer_properties. License: CC BY-SA: Attribution-ShareAlike
- Synthetic polymers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Synthetic_polymers. License: CC BY-SA: Attribution-ShareAlike
- thermoplastic. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/thermoplastic. License: CC BY-SA: Attribution-ShareAlike
- synthetic polymers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/synthetic%20polymers. License: CC BY-SA: Attribution-ShareAlike
- Polytetrafluoroethylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Polytetrafluoroethylene. License: CC BY-SA: Attribution-ShareAlike
- 100 0783. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:100_0783.JPG. License: CC BY-SA: Attribution-ShareAlike
- Organic Chemistry/Alkenes. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/Organic_Chemistry/Alkenes%23Addition_reactions. License: CC BY-SA: Attribution-ShareAlike
- Addition reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Addition_reaction. License: CC BY-SA: Attribution-ShareAlike
- addition reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/addition%20reaction. License: CC BY-SA: Attribution-ShareAlike
- addition polymerization. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/addition_polymerization. License: CC BY-SA: Attribution-ShareAlike
- 100 0783. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:100_0783.JPG. License: CC BY-SA: Attribution-ShareAlike
- Chlorine and etene addition. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Chlorine_and_etene_addition.png. License: CC BY-SA: Attribution-ShareAlike
- File:Addition reactions general overview.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Addition_reactions_general_overview.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Condensation reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Condensation_reaction. License: CC BY-SA: Attribution-ShareAlike
- condensation. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/condensation. License: CC BY-SA: Attribution-ShareAlike
- monomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/monomer. License: CC BY-SA: Attribution-ShareAlike
- condensation polymerization. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/condensation%20polymerization. License: CC BY-SA: Attribution-ShareAlike
- condensation reaction. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/condensation_reaction. License: CC BY-SA: Attribution-ShareAlike
- dehydration reaction. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dehydration_reaction. License: CC BY-SA: Attribution-ShareAlike
- 100 0783. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:100_0783.JPG. License: CC BY-SA: Attribution-ShareAlike
- Chlorine and etene addition. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Chlorine_and_etene_addition.png. License: CC BY-SA: Attribution-ShareAlike
- File:Addition reactions general overview.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Addition_reactions_general_overview.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- File:AminoacidCondensation.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:AminoacidCondensation.svg&page=1. License: Public Domain: No Known Copyright
- Nylon6 and Nylon 66. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Nylon6_and_Nylon_66.png. License: CC BY-SA: Attribution-ShareAlike
- Condensation polymerization diacid diamine. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Condensation_polymerization_diacid_diamine.svg. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter “Synthetic Organic Polymers” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
a polymer that becomes pliable or moldable above a specific temperature and returns to a solid state upon cooling
human-made polymers
reaction in which a double carbon-carbon bond forms a single carbon-carbon bond by the addition of a reactant. Typical reaction for an alkene.
A group of atoms joined by covalent bonds that are characterized by a free, unpaired electron that imparts their reactivity.
a reaction in which a compound is added to an alkene by breaking the double bond to create new single bonds to the additional compound
any reaction in which two molecules react with the resulting loss of a water molecule (or other small molecule); the formal reverse of hydrolysis
a polymerization mechanism in which monomers react to form dimers first, then trimers, longer oligomers, and eventually long chain polymers
an elimination (condensation) reaction in which the small molecule that is removed is water

