13 Classification of Matter (Solid, Liquid, Gas)
LumenLearning
Three States of Matter
The three states of matter are the distinct physical forms that matter can take: solid, liquid, and gas.
LEARNING OBJECTIVES
Describe the three states of matter.
KEY TAKEAWAYS
Key Points
- Matter can exist in one of three main states: solid, liquid, or gas.
- Solid matter is composed of tightly packed particles. A solid will retain its shape; the particles are not free to move around.
- Liquid matter is made of more loosely packed particles. It will take the shape of its container. Particles can move about within a liquid, but they are packed densely enough that volume is maintained.
- Gaseous matter is composed of particles packed so loosely that it has neither a defined shape nor a defined volume. A gas can be compressed.
Key Terms
- liquid: A substance that flows and keeps no definite shape because its molecules are loosely packed and constantly moving. It takes the shape of its container but maintains constant volume.
- gas: A substance that can only be contained if it is fully surrounded by a container (or held together by gravitational pull); a substance whose molecules have negligible intermolecular interactions and can move freely.
- solid: A substance that retains its size and shape without a container; a substance whose molecules cannot move freely except to vibrate.
The three states of matter are the three distinct physical forms that matter can take in most environments: solid, liquid, and gas. In extreme environments, other states may be present, such as plasma, Bose-Einstein condensates, and neutron stars. Further states, such as quark-gluon plasmas, are also believed to be possible. Much of the atomic matter of the universe is hot plasma in the form of rarefied interstellar medium and dense stars.
Historically, the states of matter were distinguished based on qualitative differences in their bulk properties. Solid is the state in which matter maintains a fixed volume and shape, liquid is the state in which matter adapts to the shape of its container but varies only slightly in volume, and gas is the state in which matter expands to occupy the volume and shape of its container. Each of these three classical states of matter can transition directly into either of the other two classical states.
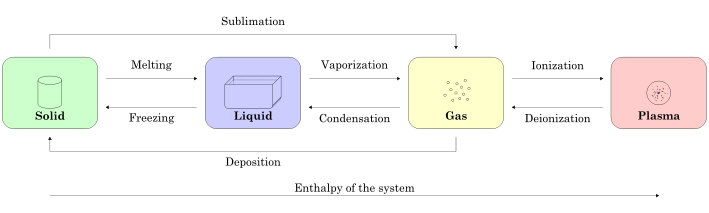
Solids
A solid’s particles are packed closely together. The forces between the particles are strong enough that the particles cannot move freely; they can only vibrate. As a result, a solid has a stable, definite shape and a definite volume. Solids can only change shape under force, as when broken or cut.
In crystalline solids, particles are packed in a regularly ordered, repeating pattern. There are many different crystal structures, and the same substance can have more than one structure. For example, iron has a body-centered cubic structure at temperatures below 912 °C and a face-centered cubic structure between 912 and 1,394 °C. Ice has 15 known crystal structures, each of which exists at a different temperature and pressure.
A solid can transform into a liquid through melting, and a liquid can transform into a solid through freezing. A solid can also change directly into a gas through a process called sublimation.
Liquids
A liquid is a fluid that conforms to the shape of its container but that retains a nearly constant volume independent of pressure. The volume is definite (does not change) if the temperature and pressure are constant. When a solid is heated above its melting point, it becomes liquid because the pressure is higher than the triple point of the substance. Intermolecular (or interatomic or interionic) forces are still important, but the molecules have enough energy to move around, which makes the structure mobile. This means that a liquid is not definite in shape but rather conforms to the shape of its container. Its volume is usually greater than that of its corresponding solid (water is a well-known exception to this rule). The highest temperature at which a particular liquid can exist is called its critical temperature.
A liquid can be converted to a gas through heating at constant pressure to the substance’s boiling point or through reduction of pressure at constant temperature. This process of a liquid changing to a gas is called evaporation.
Gases
Gas molecules have either very weak bonds or no bonds at all, so they can move freely and quickly. Because of this, not only will a gas conform to the shape of its container, it will also expand to completely fill the container. Gas molecules have enough kinetic energy that the effect of intermolecular forces is small (or zero, for an ideal gas), and they are spaced very far apart from each other; the typical distance between neighboring molecules is much greater than the size of the molecules themselves.
A gas at a temperature below its critical temperature can also be called a vapor. A vapor can be liquefied through compression without cooling. It can also exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid (or solid).
A supercritical fluid (SCF) is a gas whose temperature and pressure are greater than the critical temperature and critical pressure. In this state, the distinction between liquid and gas disappears. A supercritical fluid has the physical properties of a gas, but its high density lends it the properties of a solvent in some cases. This can be useful in several applications. For example, supercritical carbon dioxide is used to extract caffeine in the manufacturing of decaffeinated coffee.
“Phase Changes”: What does a phase change look like at the molecular level? This video takes a look at the molecular structure of solids, liquids, and gases and examines how the kinetic energy of the particles changes. The video also discusses melting, vaporization, condensation, and freezing.
KEY TERMS
- element: Any one of the simplest chemical substances that cannot be changed in a chemical reaction or by any chemical means. Made up of atoms that all have the same number of protons.
- chemical bond: Any of several attractive forces that serve to bind atoms together to form molecules.
- compound: A substance made from two or more elements. Consists of a fixed ratio of chemically bonded atoms. Has unique properties that are different from the properties of its individual elements.
Elements
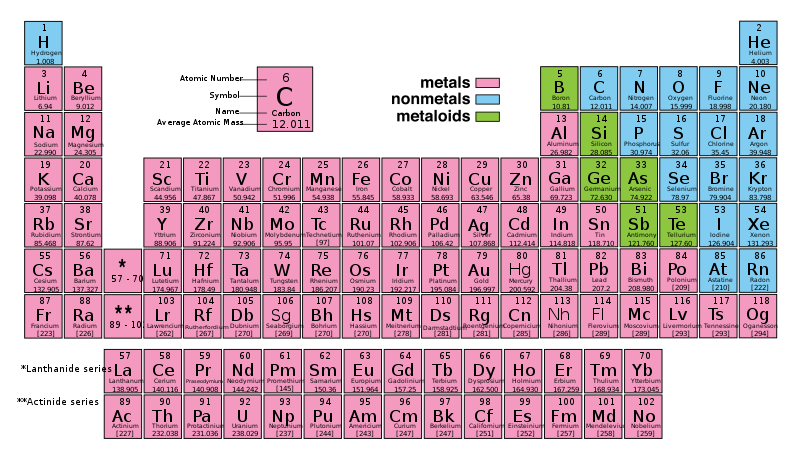
A chemical element is a pure substance that consists of one type of atom. Each atom has an atomic number, which represents the number of protons that are in the nucleus of a single atom of that element. The periodic table of elements is ordered by ascending atomic number.
The chemical elements are divided into the metals, the metalloids, and the nonmetals. Metals, typically found on the left side of the periodic table, are
- often conductive to electricity
- malleable
- shiny
- sometimes magnetic
Aluminum, iron, copper, gold, mercury, and lead are metals.
In contrast, nonmetals, found on the right side of the periodic table (to the right of the staircase), are:
- typically not conductive
- not malleable
- dull (not shiny)
- not magnetic
Examples of elemental nonmetals include carbon and oxygen.
Metalloids have some characteristics of metals and some characteristics of nonmetals. Silicon and arsenic are metalloids.
As of November 2011, 118 elements have been identified (the most recently identified was ununseptium in 2010). Of these 118 known elements, only the first 98 are known to occur naturally on Earth. The elements that do not occur naturally on Earth are the synthetic products of man-made nuclear reactions. 80 of the 98 naturally occurring elements are stable; the rest are radioactive, which means they decay into lighter elements over timescales ranging from fractions of a second to billions of years.
Hydrogen and helium are by far the most abundant elements in the universe. However, iron is the most abundant element (by mass) in the composition of the Earth, and oxygen is the most common element in the layer that is the Earth’s crust.
Although all known chemical matter is composed of these elements, chemical matter itself constitutes only about 15% of the matter in the universe. The remainder is dark matter, a mysterious substance that is not composed of chemical elements. Dark matter lacks protons, neutrons, or electrons.
Compounds
Pure samples of isolated elements are uncommon in nature. While the 98 naturally occurring elements have all been identified in mineral samples from the Earth’s crust, only a small minority of them can be found as recognizable, relatively pure minerals. Among the more common of such “native elements” are copper, silver, gold, and sulfur. Carbon is also commonly found in the form of coal, graphite, and diamonds. The noble gases (e.g., neon) and noble metals (e.g., mercury) can also be found in their pure, nonbonded forms in nature. Still, most of these elements are found in mixtures.
When two distinct elements are chemically combined—i.e., chemical bonds form between their atoms—the result is called a chemical compound. Most elements on Earth bond with other elements to form chemical compounds, such as sodium ([latex]\text{Na}[/latex]) and chloride ([latex]\text{Cl}[/latex]), which combine to form table salt ([latex]\text{NaCl}[/latex]). Water is another example of a chemical compound. The two or more component elements of a compound can be separated through chemical reactions.
Chemical compounds have a unique and defined structure, which consists of a fixed ratio of atoms held together in a defined spatial arrangement by chemical bonds. Chemical compounds can be
- molecular compounds held together by covalent bonds
- salts held together by ionic bonds
- intermetallic compounds held together by metallic bonds
- complexes held together by coordinate covalent bonds
Pure chemical elements are not considered chemical compounds, even if they consist of diatomic or polyatomic molecules (molecules that contain only multiple atoms of a single element, such as [latex]\text{H}_2[/latex] or [latex]\text{S}_8[/latex]).
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- State of matter. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/State_of_matter. License: CC BY-SA: Attribution-ShareAlike
- gas. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/gas. License: CC BY-SA: Attribution-ShareAlike
- liquid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/liquid. License: CC BY-SA: Attribution-ShareAlike
- solid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/solid. License: CC BY-SA: Attribution-ShareAlike
- “Phase Changes” – YouTube. Located at: http://www.youtube.com/watch?v=EZHmUTmJtF8. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Physics matter state transition 1 en. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Physics_matter_state_transition_1_en.svg. License: CC BY-SA: Attribution-ShareAlike
- Chemical substance. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_substance. License: CC BY-SA: Attribution-ShareAlike
- Mixture. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Mixture. License: CC BY-SA: Attribution-ShareAlike
- mixture. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/mixture. License: CC BY-SA: Attribution-ShareAlike
- substance. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/substance. License: CC BY-SA: Attribution-ShareAlike
- “Phase Changes” – YouTube. Located at: http://www.youtube.com/watch?v=EZHmUTmJtF8. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Physics matter state transition 1 en. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Physics_matter_state_transition_1_en.svg. License: CC BY-SA: Attribution-ShareAlike
- “Chemistry 1.2 Classifying Matter (Part 1 of 3)” – YouTube. Located at: http://www.youtube.com/watch?v=ZZYjleLadlc. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Schwefel 01. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Schwefel_01.jpg. License: CC BY-SA: Attribution-ShareAlike
- Chemical element. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_element. License: CC BY-SA: Attribution-ShareAlike
- Chemical compound. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_compound. License: CC BY-SA: Attribution-ShareAlike
- element. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/element. License: CC BY-SA: Attribution-ShareAlike
- compound. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/compound. License: CC BY-SA: Attribution-ShareAlike
- chemical bond. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/chemical_bond. License: CC BY-SA: Attribution-ShareAlike
- “Phase Changes” – YouTube. Located at: http://www.youtube.com/watch?v=EZHmUTmJtF8. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Physics matter state transition 1 en. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Physics_matter_state_transition_1_en.svg. License: CC BY-SA: Attribution-ShareAlike
- “Chemistry 1.2 Classifying Matter (Part 1 of 3)” – YouTube. Located at: http://www.youtube.com/watch?v=ZZYjleLadlc. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Schwefel 01. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Schwefel_01.jpg. License: CC BY-SA: Attribution-ShareAlike
- “Chemistry 1.2 Classifying Matter (Part 2 of 3)” – YouTube. Located at: http://www.youtube.com/watch?v=SoFywULNF3s. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
This chapter is an adaptation of the chapter “Classification of Matter” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
A substance that flows and keeps no definite shape because its molecules are loosely packed and constantly moving. It takes the shape of its container but maintains constant volume.
A substance that can only be contained if it is fully surrounded by a container (or held together by gravitational pull); a substance whose molecules have negligible intermolecular interactions and can move freely.
A substance that retains its size and shape without a container; a substance whose molecules cannot move freely except to vibrate.
Any one of the simplest chemical substances that cannot be decomposed in a chemical reaction or by any chemical means, and are made up of atoms all having the same number of protons.
Any of several attractive forces that serve to bind atoms together to form molecules.
A substance made from two or more elements. Consists of a fixed ratio of chemically bonded atoms. Has unique properties that are different from the properties of its individual elements.

