48 Electronegativity
LumenLearning
Electronegativity
Electronegativity is the tendency of an atom/molecule to attract electrons.
KEY TAKEAWAYS
Key Points
- An atom ‘s electronegativity is affected by both the element ‘s atomic number and its size.
- The higher its electronegativity, the more an element attracts electrons.
Key Terms
- electronegativity: A chemical property that describes the tendency of an atom to attract electrons (or electron density) toward itself.
Electronegativity
Electronegativity is a property that describes the tendency of an atom to attract electrons (or electron density) toward itself. An atom’s electronegativity is affected by both its atomic number and the size of the atom. The higher its electronegativity, the more an element attracts electrons. The opposite of electronegativity is electropositivity, which is a measure of an element’s ability to donate electrons.
Electronegativity is not directly measured, but is instead calculated based on experimental measurements of other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the calculated electronegativity values, all methods show the same periodic trend among the elements.
Electronegativity, as it is usually calculated, is not strictly a property of an atom, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property; that is to say, similar values will be valid in a variety of situations.
On the most basic level, electronegativity is determined by factors such as the nuclear charge and the number/location of other electrons present in the atomic shells. The nuclear charge is important because the more protons an atom has, the more “pull” it will have on negative electrons. Where electrons are in space is a contributing factor because the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result they will experience less positive charge; this is due to their increased distance from the nucleus, and because the other electrons in the lower-energy core orbitals will act to shield the valence electrons from the positively charged nucleus.
The most commonly used method of calculation for electronegativity was proposed by Linus Pauling. This method yields a dimensionless quantity, commonly referred to as the Pauling scale, with a range from 0.7 to 4. If we look at the periodic table without the inert gases, electronegativity is greatest in the upper right and lowest at the bottom left.
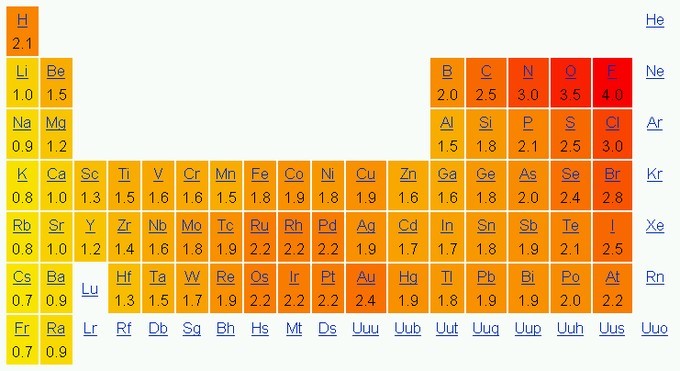
Hence, fluorine ([latex]\text{F}[/latex]) is the most electronegative of the elements, while francium ([latex]\text{Fr}[/latex]) is the least electronegative.
Bond Polarity
Molecular polarity is dependent on the presence of polar covalent bonds and the molecule’s three-dimensional structure.
LEARNING OBJECTIVES
Apply knowledge of bond polarity and molecular geometry to identify the dipole moment of molecules
KEY TAKEAWAYS
Key Points
- When non-identical atoms are covalently bonded, the electron pair will be attracted more strongly to the atom that has the higher electronegativity. This results in a polar covalent bond.
- Polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment.
- A polar molecule acts as an electric dipole that can interact with electric fields that are created artificially, or that arise from nearby ions or polar molecules.
- The dipole moment [latex]\mu[/latex] that corresponds to an individual bond is given by the product of the quantity of charge, q, and the bond length r: [latex]\mu = qr[/latex].
Key Terms
- Bond polarity: A covalent bond is polar if one atom is more electronegative than its bonding partner, resulting in a net dipole moment between the two atoms.
- dipole moment: A measure of the polarity of a covalent bond or of an entire molecule. It is the product of the charge on either pole of the dipole and the distance separating them.
- Molecular polarity: A molecule is polar if it has a net dipole moment, which depends on the existence of polar covalent bonds and the molecule’s three-dimensional structure or geometry.
Bond vs Molecular Polarity
Polarity refers to the separation of charge that creates permanent positive and negative ‘electric poles.’ This concept can be applied in two contexts:
- Bond polarity: when atoms from different elements are covalently bonded, the shared pair of electrons will be attracted more strongly to the atom with the higher electronegativity. As a result, the electrons will not be shared equally. Such bonds are said to be ‘polar’ and possess partial ionic character.
- Molecular polarity: when an entire molecule, which can be made out of several covalent bonds, has a net polarity, with one end having a higher concentration of negative charge and another end having a surplus of positive charge. A polar molecule acts as an electric dipole which can interact with electric fields that are created artificially, or that arise from interactions with nearby ions or other polar molecules.
Dipole Moment
Dipoles are conventionally represented as arrows pointing in the direction of the negative end. The strength of a dipole’s interaction with an electric field is given by the electric dipole moment of the bond or molecule. The dipole moment is calculated by evaluating the product of the magnitude of separated charge, q, and the bond length, r:
[latex]\mu = q r[/latex]
In SI units, q is expressed in coulombs and r in meters, so μ has the dimensions of [latex]C \cdot m[/latex]. If two charges of magnitude +1 and -1 are separated by a typical bond length of 100 pm, then:
[latex]\mu = (1.6022 \times 10^{-19} C) \times (10^{-10} m) = 1.6 \times 10^{-29} C \cdot m = 4.8 D[/latex]
The Debye unit, D, is commonly used to express dipole moments.
Determining a Molecule’s Dipole Moment
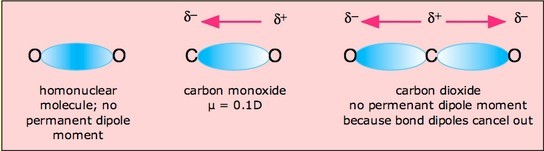
In molecules containing more than one polar bond, the molecular dipole moment is just the vector addition of the individual bond dipole moments. Being vectors, these can reinforce or cancel each other depending on the geometry of the molecule. Therefore, it is possible for molecules containing polar bonds to be nonpolar overall, as in the example of carbon dioxide.
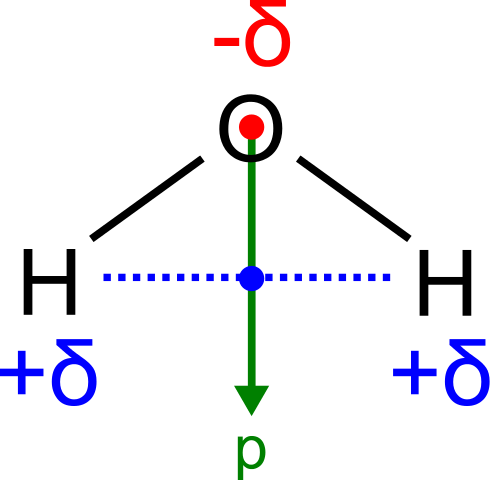
[latex]\text{H}_2\text{O}[/latex], by contrast, has a very large molecular dipole moment which results from the two polar H–O bonds forming an angle of 104.5° between them. The water molecule, therefore, is polar.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- electronegativity. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/electronegativity. License: CC BY-SA: Attribution-ShareAlike
- Polar Covalence. Provided by: Steve Lower’s Website. Located at: http://www.chem1.com/acad/webtext/chembond/cb04.html#SEC1. License: CC BY-SA: Attribution-ShareAlike
- Provided by: AskApache. Located at: http://nongnu.askapache.com/fhsst/Chemistry_Grade_10-12.pdf. License:
- Electronegativity. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Electronegativity. License: CC BY-SA: Attribution-ShareAlike
- Oxidation number. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Oxidation_number. License: CC BY-SA: Attribution-ShareAlike
- Provided by: African Virtual University. Located at: http://oer.avu.org/bitstream/handle/123456789/42/Introductory%20Chemistry%201.pdf?sequence=4. License: CC BY: Attribution
- Oxidation State. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Oxidation%20State. License: CC BY-SA: Attribution-ShareAlike
- oxidation number. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/oxidation_number. License: CC BY-SA: Attribution-ShareAlike
- Taula periòdica electronegativitat. Provided by: Wikimedia. Located at: http://commons.wikimedia.org/wiki/File:Taula_peri%C3%B2dica_electronegativitat.png. License: CC BY-SA: Attribution-ShareAlike
- Polar Covalence. Provided by: Steve Lower’s Website. Located at: http://www.chem1.com/acad/webtext/chembond/cb04.html#SEC1. License: CC BY-SA: Attribution-ShareAlike
- Bond polarity. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Bond_polarity. License: CC BY-SA: Attribution-ShareAlike
- Electronegativity. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Electronegativity. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- polarity. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/polarity. License: CC BY-SA: Attribution-ShareAlike
- dipole moment. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole_moment. License: CC BY-SA: Attribution-ShareAlike
- Taula periòdica electronegativitat. Provided by: Wikimedia. Located at: http://commons.wikimedia.org/wiki/File:Taula_peri%C3%B2dica_electronegativitat.png. License: CC BY-SA: Attribution-ShareAlike
- 490px-Dipole_Water.svg.png. Provided by: Wikimedia Commons. Located at: https://commons.wikimedia.org/wiki/File:Dipole_Water.svg. License: CC BY: Attribution
- Polar Covalence. Provided by: Steve Lower. Located at: http://www.chem1.com/acad/webtext/chembond/cb04.html#SEC1. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter “Electronegativity” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
tendency of an atom to attract electrons in a bond to itself
property of a molecule that describes the separation of charge determined by the sum of the individual bond moments based on the molecular structure

