43 The Ionic Bond
LumenLearning
Ionic Bonding and Electron Transfer
An ionic bond results from the transfer of an electron from a metal atom to a non-metal atom.
LEARNING OBJECTIVES
Identify the key features of ionic bonds
KEY TAKEAWAYS
Key Points
- Ionic bonds are formed between cations and anions.
- A cation is formed when a metal ion loses a valence electron while an anion is formed when a non-metal gains a valence electron. They both achieve a more stable electronic configuration through this exchange.
- Ionic solids form crystalline lattices, or repeating patterns of atoms, with high melting points, and are typically soluble in water.
Key Terms
- electrolyte: An ionic compound which dissolves in H2O, making the resulting solution capable of conducting electricity.
- electronegativity: The tendency of an atom to attract electrons to itself.
- cation: A positively charged ion.
- anion: A negatively charged ion.
Ionic Bonds
Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable, noble gas electronic configuration for both atoms involved. An ionic bond is based on attractive electrostatic forces between two ions of opposite charge.
Cations and Anions
Ionic bonds involve a cation and an anion. The bond is formed when an atom, typically a metal, loses an electron or electrons, and becomes a positive ion, or cation. Another atom, typically a non-metal, is able to acquire the electron(s) to become a negative ion, or anion.
One example of an ionic bond is the formation of sodium fluoride, NaF, from a sodium atom and a fluorine atom. In this reaction, the sodium atom loses its single valence electron to the fluorine atom, which has just enough space to accept it. The ions produced are oppositely charged and are attracted to one another due to electrostatic forces.
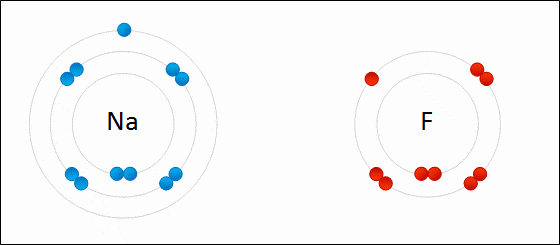
At the macroscopic scale, ionic compounds form lattices, are crystalline solids under normal conditions, and have high melting points. Most of these solids are soluble in [latex]\text{H}_2\text{O}[/latex] and conduct electricity when dissolved. The ability to conduct electricity in solution is why these substances are called electrolytes. Table salt, [latex]\text{NaCl}[/latex], is a good example of this type of compound.
Ionic bonds differ from covalent bonds. Both types result in the stable electronic states associated with the noble gases. However, in covalent bonds, the electrons are shared between the two atoms. All ionic bonds have some covalent character, but the larger the difference in electronegativity between the two atoms, the greater the ionic character of the interaction.
Ionic Bonding – YouTube: In this video, Paul Andersen explains how ionic solids form when cations and anions are attracted.
Lattice Energy
Lattice energy is a measure of the bond strength in an ionic compound.
LEARNING OBJECTIVES
Describe lattice energy and the factors that affect it
KEY TAKEAWAYS
Key Points
- Lattice energy is defined as the energy required to separate a mole of an ionic solid into gaseous ions.
- Lattice energy cannot be measured empirically, but it can be calculated using electrostatics or estimated using the Born-Haber cycle.
- Two main factors that contribute to the magnitude of the lattice energy are the charge and radius of the bonded ions.
Key Terms
- exothermic reaction: A process which releases heat into its surroundings.
- lattice energy: The amount of energy released upon formation of a crystalline ionic solid from gaseous ions.
Definition of Lattice Energy
Lattice energy is an estimate of the bond strength in ionic compounds. It is defined as the heat of formation for ions of opposite charge in the gas phase to combine into an ionic solid. As an example, the lattice energy of sodium chloride, [latex]\text{NaCl}[/latex], is the energy released when gaseous [latex]\text{Na}^+[/latex] and [latex]\text{Cl}^-[/latex] ions come together to form a lattice of alternating ions in the [latex]\text{NaCl}[/latex] crystal.
[latex]\text{Na}^+(g) + \text{Cl}^-(g) \rightarrow \text{NaCl}(s) \ \ \ \ \ \ \ \Delta H = -787.3 \text{ kJ/mol}[/latex]
The negative sign of the energy is indicative of an exothermic reaction.
Alternatively, lattice energy can be thought of as the energy required to separate a mole of an ionic solid into the gaseous form of its ions (that is, the reverse of the reaction shown above).
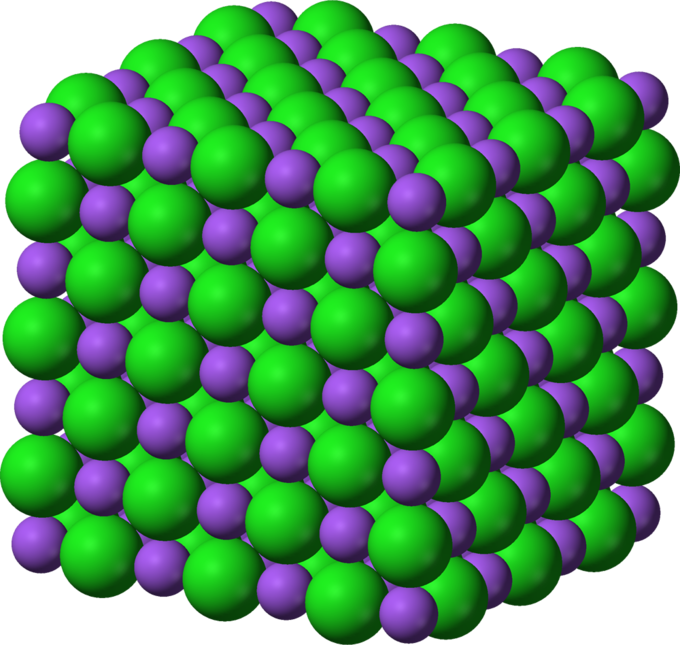
Alternatively, lattice energy can be thought of as the energy required to separate a mole of an ionic solid into the gaseous form of its ions (that is, the reverse of the reaction shown above).
Lattice energy cannot be determined experimentally due to the difficulty in isolating gaseous ions. The energy value can be estimated using the Born-Haber cycle, or it can be calculated theoretically with an electrostatic examination of the crystal structure.
Factors Affecting Lattice Energy
In 1918, Born and Lande presented the following model for lattice energy:
[latex]E = - \frac{N_A M z^+ z^- e^2}{4 \pi \epsilon _0 r_0} (1 - \frac{1}{n})[/latex]
In this equation, NA is Avogadro’s constant; M is the Madelung constant, which depends on the crystal geometry; z+ is the charge number of the cation; z– is the charge number of the anion; e is the elementary charge of the electron; n is the Born exponent, a characteristic of the compressibility of the solid; [latex]\epsilon _o[/latex] is the permittivity of free space; and r0 is the distance to the closest ion.
This model emphasizes two main factors that contribute to the lattice energy of an ionic solid: the charge on the ions, and the radius, or size, of the ions. The effect of those factors is:
- as the charge of the ions increases, the lattice energy increases
- as the size of the ions increases, the lattice energy decreases
Lattice energies are also important in predicting the solubility of ionic solids in [latex]\text{H}_2\text{O}[/latex]. Ionic compounds with smaller lattice energies tend to be more soluble in [latex]\text{H}_2\text{O}[/latex].
Lattice Energies – Chemistry Tutorial: This tutorial covers lattice energy and how to compare the relative lattice energies of different ionic compounds.
Formulas of Ionic Compounds
Ionic formulas must satisfy the noble gas configurations for the constituent ions and the product compound must be electrically neutral.
LEARNING OBJECTIVES
Apply knowledge of ionic bonding to predict the formula of ionic compounds
KEY TAKEAWAYS
Key Points
- The charge on the cations and anions in an ionic compound can be determined by the loss or gain of valence electrons necessary in order to achieve stable, noble gas electronic configurations.
- The number of cations and anions that are combined in an ionic compound is the simplest ratio of whole integers that can be combined to reach electrical neutrality.
- The cation precedes the anion in both the written form and the formula.
Key Terms
- noble gas: Any of the elements of group 18 of the periodic table, which are monatomic and, with very limited exceptions, inert, or non-reactive.
- electrically neutral: A net charge of zero, which occurs when an atom or molecule/compound has no surplus or deficit of electrons.
- empirical formula: The simplest whole-number ratio between elements in a formula of a compound.
- polyatomic ion: An ion composed of several atoms.
Ionic Compounds
An ionic bond is formed through the transfer of one or more valence electrons, typically from a metal to a non-metal, which produces a cation and an anion that are bound together by an attractive electrostatic force. On a macroscopic scale, ionic compounds, such as sodium chloride ([latex]\text{NaCl}[/latex]), form a crystalline lattice and are solids at normal temperatures and pressures.
The charge on the cations and anions is determined by the number of electrons required to achieve stable noble gas electronic configurations. The ionic composition is then defined by the requirement that the resulting compound be electrically neutral overall.
For example, to combine magnesium ([latex]\text{Mg}[/latex]) and bromine ([latex]\text{Br}[/latex]) to get an ionic compound, we first note the electronic configurations of these atoms (valence level in indicated in italics):
Mg: 1s22s22p63s2
Br: 1s22s22p63s23p63d104s24p5
In order to achieve noble gas configurations, the magnesium atom needs to lose its two valence electrons, while the bromine atom, which has 7 valence electrons, requires one additional electron to fill its outer shell. Therefore, for the resulting compound to be neutral, two bromine anions must combine with one magnesium cation to form magnesium bromide ([latex]\text{MgBr}_2[/latex]). In addition, though any ratio of 2 bromine atoms to 1 magnesium atom will satisfy the two requirements above, the formula for ionic compounds is typically presented as the empirical formula, or the simplest whole-number ratio of atoms with positive integers.
Note that the cation always precedes the anion both in written form and in formulas. In the written form, while the cation name is generally the same as the element, the suffix of single-atom anions is changed to –ide, as in the case of sodium chloride. If the anion is a polyatomic ion, its suffix can vary, but is typically either –ate or –ite,as in the cases of sodium phosphate and calcium nitrite, depending on the identity of the ion.
More examples:
- lithium fluoride: [latex]\text{Li}^+[/latex] and [latex]\text{F}^-[/latex] combine to form [latex]\text{LiF}[/latex]
- calcium chloride: [latex]\text{Ca}^{2+}[/latex] and [latex]\text{Cl}^-[/latex] combine to form [latex]\text{CaCl}_2[/latex]
- iron (II) oxide: [latex]\text{Fe}^{2+}[/latex] and [latex]\text{O}^{2-}[/latex] combine to form [latex]\text{FeO}[/latex]
- aluminum sulfide: [latex]\text{Al}^{3+}[/latex] and [latex]\text{S}^{2-}[/latex] combine to form [latex]\text{Al}_2\text{S}_3[/latex]
- sodium sulfate: [latex]\text{Na}^+[/latex] and [latex]\text{SO}_4^{2-}[/latex] combine to form [latex]\text{Na}_2\text{SO}_4[/latex]
- ammonium phosphate: [latex]\text{NH}^{4+}[/latex] and [latex]\text{PO}_4^{3-}[/latex] combine to form [latex]\text{(NH}_4\text{)}_3\text{PO}_4[/latex]
- potassium chlorite: [latex]\text{K}^+[/latex] and [latex]\text{ClO}_2^-[/latex] combine to form [latex]\text{KClO}_2[/latex]
Video Summary
Chemistry 5.3 Formula Writing: Ionic Compounds – YouTube: A lesson on writing formulas for binary ionic compounds as well as ionic compounds containing polyatomic ions. The cross-over method is demonstrated.
Ionic vs Covalent Bond Character
Ionic bonds can have some covalent character.
LEARNING OBJECTIVES
Discuss the idea that, in nature, bonds exhibit characteristics of both ionic and covalent bonds
KEY TAKEAWAYS
Key Points
- Ionic bonding is presented as the complete transfer of valence electrons, typically from a metal to a non-metal.
- In reality, electron density remains shared between the constituent atoms, meaning all bonds have some covalent character.
- The ionic or covalent nature of a bond is determined by the relative electronegativities of the atoms involved.
Key Terms
- polar covalent bond: A covalent bond that has a partial ionic character to it, as a result of the difference in electronegativity between the two bonding atoms.
- electronegativity: A measure of the tendency of an atom to attract electrons to itself.
- covalent character: The partial sharing of electrons between atoms that have an ionic bond.
Ionic vs Covalent Bonding
Chemical compounds are frequently classified by the bonds between constituent atoms. There are multiple kinds of attractive forces, including covalent, ionic, and metallic bonds. Ionic bonding models are generally presented as the complete loss or gain of one or more valence electrons from a metal to a nonmetal, resulting in cations and anions that are held together by attractive electrostatic forces.
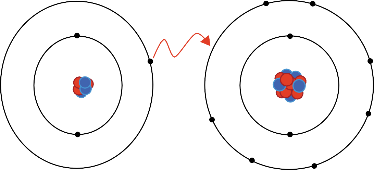
In reality, the bond between these atoms is more complex than this model illustrates. The bond formed between any two atoms is not a purely ionic bond. All bonding interactions have some covalent character because the electron density remains shared between the atoms. The degree of ionic versus covalent character of a bond is determined by the difference in electronegativity between the constituent atoms. The larger the difference, the more ionic the nature of the bond. In the conventional presentation, bonds are designated as ionic when the ionic aspect is greater than the covalent aspect of the bond. Bonds that fall in between the two extremes, having both ionic and covalent character, are classified as polar covalent bonds. Such bonds are thought of as consisting of partially charged positive and negative poles.
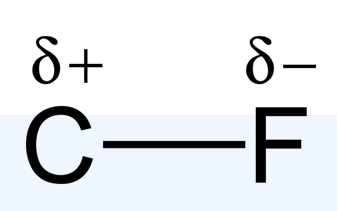
Though ionic and covalent character represent points along a continuum, these designations are frequently useful in understanding and comparing the macroscopic properties of ionic and covalent compounds. For example, ionic compounds typically have higher boiling and melting points, and they are also usually more soluble in water than covalent compounds.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Ionic bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ionic_bond. License: CC BY-SA: Attribution-ShareAlike
- anion. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/anion. License: CC BY-SA: Attribution-ShareAlike
- cation. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/cation. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- Ionic Bonding – YouTube. Located at: http://www.youtube.com/watch?v=hiyTfhjeF_U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Lattice energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Lattice_energy. License: CC BY-SA: Attribution-ShareAlike
- Born-Haber Cycle. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Born-Haber_Cycle. License: CC BY-SA: Attribution-ShareAlike
- exothermic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/exothermic. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- Ionic Bonding – YouTube. Located at: http://www.youtube.com/watch?v=hiyTfhjeF_U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Lattice Energies – Chemistry Tutorial. Located at: http://www.youtube.com/watch?v=EwnU8RalZOw. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- noble gas. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/noble_gas. License: CC BY-SA: Attribution-ShareAlike
- Ionic compound. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ionic_compound. License: CC BY-SA: Attribution-ShareAlike
- IUPAC nomenclature. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/IUPAC_nomenclature. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- Ionic Bonding – YouTube. Located at: http://www.youtube.com/watch?v=hiyTfhjeF_U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Lattice Energies – Chemistry Tutorial. Located at: http://www.youtube.com/watch?v=EwnU8RalZOw. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Chemistry 5.3 Formula Writing: Ionic Compounds – YouTube. Located at: http://www.youtube.com/watch?v=bvFylpHrJJY. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Ionic bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ionic_bond. License: CC BY-SA: Attribution-ShareAlike
- covalent. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/covalent. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- Ionic Bonding – YouTube. Located at: http://www.youtube.com/watch?v=hiyTfhjeF_U. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Lattice Energies – Chemistry Tutorial. Located at: http://www.youtube.com/watch?v=EwnU8RalZOw. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Chemistry 5.3 Formula Writing: Ionic Compounds – YouTube. Located at: http://www.youtube.com/watch?v=bvFylpHrJJY. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Wikipedia. Provided by: Wikipedia. Located at: http://www.wikipedia.org. License: CC BY-SA: Attribution-ShareAlike
- Carbon-fluorine-bond-polarity-2D-black. Provided by: Wikimedia Commons. Located at: https://commons.wikimedia.org/wiki/File:Carbon-fluorine-bond-polarity-2D-black.png. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter "The Ionic Bond" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
tendency of an atom to attract electrons in a bond to itself
Ions that are positively charged because they have more protons than electrons.
Ions that are negatively charged because they have more electrons than protons.
(also, inert gas) element in group 18
ion composed of more than one atom
covalent bond between atoms of different electronegativities; a covalent bond with a positive end and a negative end

