45 The Covalent Bond
LumenLearning
Comparison between Covalent and Ionic Compounds
Covalent and ionic compounds have distinct physical properties.
LEARNING OBJECTIVES
Identify element pairs which are likely to form ionic or covalent bonds
KEY TAKEAWAYS
Key Points
- Ionic compounds are formed from strong electrostatic interactions between ions, which result in higher melting points and electrical conductivity compared to covalent compounds.
- Covalent compounds have bonds where electrons are shared between atoms. Due to the sharing of electrons, they exhibit characteristic physical properties that include lower melting points and electrical conductivity compared to ionic compounds.
Key Terms
- valence electrons: Electrons in the outermost principal energy (valence) level of an atom that can participate in the formation of chemical bonds with other atoms.
- octet rule: Atoms lose, gain, or share electrons in order to have a full valence level of eight electrons. Hydrogen and helium are exceptions because they can hold a maximum of two valence electrons.
- electronegativity: The tendency of an atom or molecule to attract electrons and form bonds.
Two Classes of Compounds
Compounds are defined as substances containing two or more different chemical elements. They have distinct chemical structures characterized by a fixed ratio of atoms held together by chemical bonds. Here, we discuss two classes of compounds based on the bond type that holds the atoms together: ionic and covalent.
Covalent Compounds
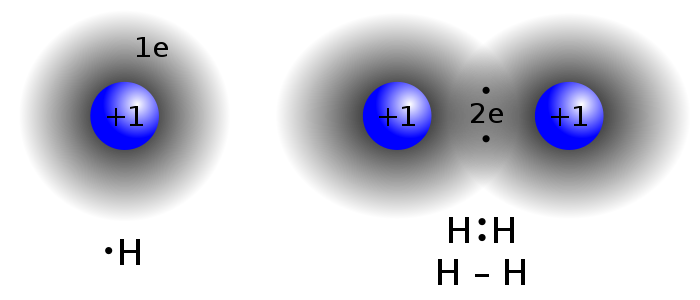
Covalent bonds are characterized by the sharing of electrons between two or more atoms. These bonds mostly occur between nonmetals or between two of the same (or similar) elements.Two atoms with similar electronegativity will not exchange an electron from their outermost shell; the atoms instead share electrons so that their valence electron shell is filled.
Examples of compounds that contain only covalent bonds are methane ([latex]\text{CH}_4[/latex]), carbon monoxide ([latex]\text{CO}[/latex]), and iodine monobromide ([latex]\text{IBr}[/latex]).
Ionic Compounds
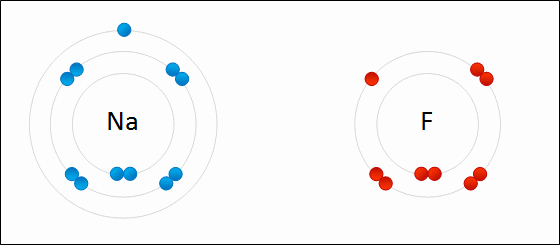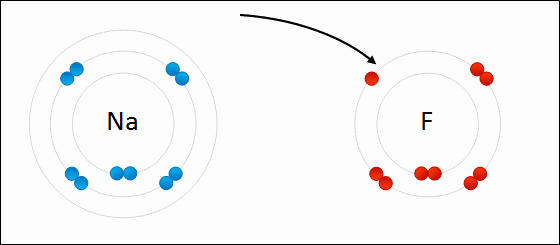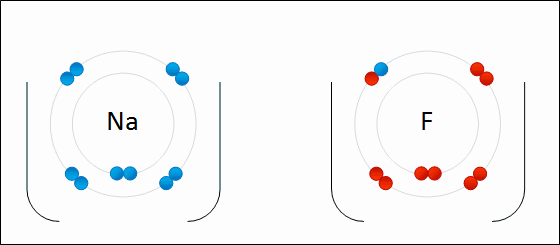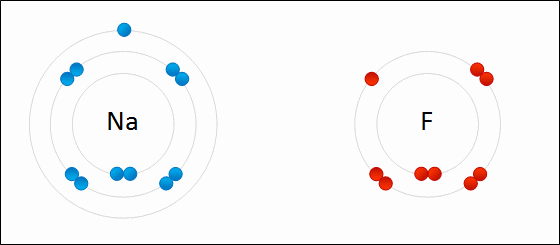
Ionic bonding occurs when there is a large difference in electronegativity between two atoms. This large difference leads to the loss of an electron from the less electronegative atom and the gain of that electron by the more electronegative atom, resulting in two ions. These oppositely charged ions feel an attraction to each other, and this electrostatic attraction constitutes an ionic bond.
Ionic bonding occurs between a nonmetal, which acts as an electron acceptor, and a metal, which acts as an electron donor. Metals have few valence electrons, whereas nonmetals have closer to eight valence electrons; to easily satisfy the octet rule, the nonmetal will accept an electron donated by the metal. More than one electron can be donated and received in an ionic bond.
Some examples of compounds with ionic bonding include [latex]\text{NaCl}[/latex], [latex]\text{KI}[/latex], [latex]\text{MgCl}_2[/latex].
Effect on Physical Properties
Covalent and ionic compounds can be differentiated easily because of their different physical properties based on the nature of their bonding. Here are some differences:
- At room temperature and normal atmospheric pressure, covalent compounds may exist as a solid, a liquid, or a gas, whereas ionic compounds exist only as solids.
- Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an electrically conductive solution. In contrast, covalent compounds do not exhibit any electrical conductivity, either in pure form or when dissolved in water.
- Ionic compounds exist in stable crystalline structures. Therefore, they have higher melting and boiling points compared to covalent compounds.
Single Covalent Bonds
Single covalent bonds are sigma bonds, which occur when one pair of electrons is shared between atoms.
LEARNING OBJECTIVES
Identify the four orbital types used in covalent bond formation
KEY TAKEAWAYS
Key Points
- Covalent bonds occur when electrons are shared between two atoms. A single covalent bond is when only one pair of electrons is shared between atoms.
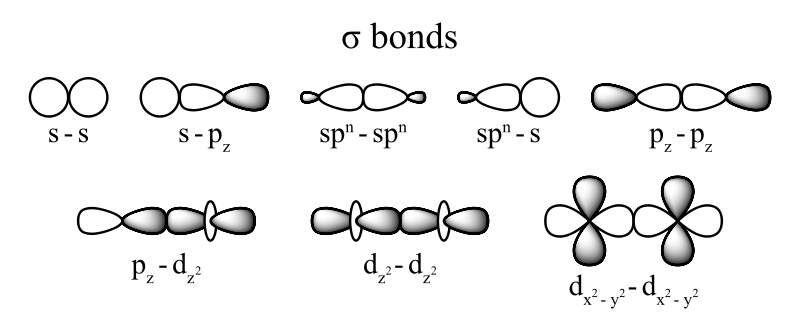
- A sigma bond is the strongest type of covalent bond, in which the atomic orbitals directly overlap between the nuclei of two atoms.
- Sigma bonds can occur between any kind of atomic orbitals; the only requirement is that the atomic orbital overlap happens directly between the nuclei of atoms.
Key Terms
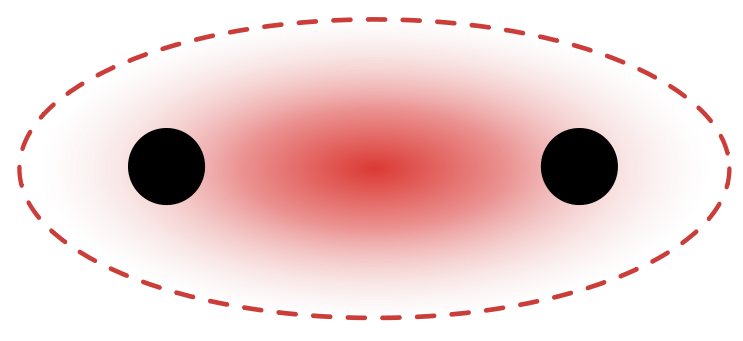
- sigma bond: A covalent bond whose electron density is concentrated in the region directly between the nuclei.
- covalent bond: A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
- atomic orbital: A region in space around the atom’s nucleus where there is a probability of finding an electron.
Hierarchical Structure of the Atom
There are four hierarchical levels that describe the position and energy of the electrons an atom has. Here they are listed along with some of the possible values (or letters) they can have:
- Principal energy levels (1, 2, 3, etc.)
- Sublevels (s, p, d, f)
- Orbitals
- Electrons
Principal energy levels are made out of sublevels, which are in turn made out of orbitals, in which electrons are found.
Atomic Orbitals
An atomic orbital is defined as the probability of finding an electron in an area around an atom’s nucleus. Generally, orbital shapes are drawn to describe the region in space in which electrons are likely to be found. This is referred to as “electron density.”
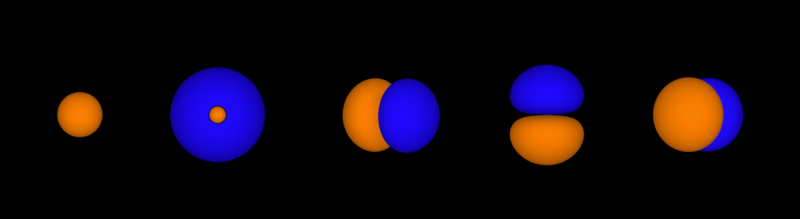
Atomic Orbitals
Covalent bonding occurs when two atomic orbitals come together in close proximity and their electron densities overlap. The strongest type of covalent bonds are sigma bonds, which are formed by the direct overlap of orbitals from each of the two bonded atoms. Regardless of the atomic orbital type, sigma bonds can occur as long as the orbitals directly overlap between the nuclei of the atoms.
Single covalent bonds occur when one pair of electrons is shared between atoms as part of a molecule or compound. A single covalent bond can be represented by a single line between the two atoms. For instance, the diatomic hydrogen molecule, [latex]\text{H}_2[/latex], can be written as [latex]\text{H-H}[/latex] to indicate the single covalent bond between the two hydrogen atoms.
Double and Triple Covalent Bonds
Double and triple bonds, comprised of sigma and pi bonds, increase the stability and restrict the geometry of a compound.
LEARNING OBJECTIVES
Describe the types of orbital overlap that occur in single, double, and triple bonds
KEY TAKEAWAYS
Key Points
- Double and triple covalent bonds are stronger than single covalent bonds and they are characterized by the sharing of four or six electrons between atoms, respectively.
- Double and triple bonds are comprised of sigma bonds between hybridized orbitals, and pi bonds between unhybridized p orbitals. Double and triple bonds offer added stability to compounds, and restrict any rotation around the bond axis.
- Bond lengths between atoms with multiple bonds are shorter than in those with single bonds.
Key Terms
- bond strength: Directly related to the amount of energy required to break the bond between two atoms. The more energy required, the stronger the bond is said to be.
- bond length: The distance between the nuclei of two bonded atoms. It can be experimentally determined.
- orbital hybridization: The concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties and geometries.
- atomic orbitals: The physical region in space around the nucleus where an electron has a probability of being.
Double and Triple Covalent Bonds
Covalent bonding occurs when electrons are shared between atoms. Double and triple covalent bonds occur when four or six electrons are shared between two atoms, and they are indicated in Lewis structures by drawing two or three lines connecting one atom to another. It is important to note that only atoms with the need to gain or lose at least two valence electrons through sharing can participate in multiple bonds.
Physical Properties of Covalent Molecules
The covalent bonding model helps predict many of the physical properties of compounds.
LEARNING OBJECTIVES
Discuss the qualitative predictions of covalent bond theory on the boiling and melting points, bond length and strength, and conductivity of molecules
KEY TAKEAWAYS
Key Points
- The Lewis theory of covalent bonding says that the bond strength of double bonds is twice that of single bonds, which is not true.
- General physical properties that can be explained by the covalent bonding model include boiling and melting points, electrical conductivity, bond strength, and bond length.
Key Terms
- bond length: The distance between the nuclei of two bonded atoms. It can be experimentally determined.
- intermolecular forces: Attractive forces or interactions between different molecules in a sample of a substance. The strength of these interactions is an important factor that determines the substance’s physical properties.
- bond strength: Directly related to the amount of energy required to break the bond between two atoms. The more energy required, the stronger the bond is said to be.
- octet rule: Atoms lose, gain, or share electrons in order to have a full valence shell of eight electrons. Hydrogen is an exception because it can hold a maximum of two electrons in its valence level.
First described by Gilbert Lewis, a covalent bond occurs when electrons of different atoms are shared between the two atoms. These cases of electron sharing can be predicted by the octet rule. The octet rule is a chemical rule that generalizes that atoms of low atomic number (< 20) will combine in a way that results in their having 8 electrons in their valence shells. Having 8 valence electrons is favorable for stability and is similar to the electron configuration of the inert noble gases. In a covalent bond, the shared electrons contribute to each atom’s octet and thus enhance the stability of the compound.
The Lewis bonding theory can explain many properties of compounds. For example, the theory predicts the existence of diatomic molecules such as hydrogen, [latex]\text{H}_2[/latex], and the halogens ([latex]\text{F}_2[/latex], [latex]\text{Cl}_2[/latex], [latex]\text{Br}_2[/latex], [latex]\text{I}_2[/latex]). A H atom needs one additional electron to fill its valence level, and the halogens need one more electron to fill the octet in their valence levels. Lewis bonding theory states that these atoms will share their valence electrons, effectively allowing each atom to create its own octet.
Several physical properties of molecules/compounds are related to the presence of covalent bonds:
- Covalent bonds between atoms are quite strong, but attractions between molecules/compounds, or intermolecular forces, can be relatively weak. Covalent compounds generally have low boiling and melting points, and are found in all three physical states at room temperature.
- Covalent compounds do not conduct electricity; this is because covalent compounds do not have charged particles capable of transporting electrons.
- Lewis theory also accounts for bond length; the stronger the bond and the more electrons shared, the shorter the bond length is.
However, the Lewis theory of covalent bonding does not account for some observations of compounds in nature. The theory predicts that with more shared electrons, the bond between the two atoms should be stronger. According to the theory, triple bonds are stronger than double bonds, and double bonds are stronger than single bonds. This is true. However, the theory implies that the bond strength of double bonds is twice that of single bonds, which is not true. Therefore, while the covalent bonding model accounts for many physical observations, it does have its limitations.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- octet rule. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/octet+rule. License: CC BY-SA: Attribution-ShareAlike
- Ionic compound. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ionic_compound. License: CC BY-SA: Attribution-ShareAlike
- Covalent bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Covalent_bond. License: CC BY-SA: Attribution-ShareAlike
- valence electrons. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/valence%20electrons. License: CC BY-SA: Attribution-ShareAlike
- electronegativity. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/electronegativity. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- File:Covalent bond hydrogen.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Covalent_bond_hydrogen.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Atomic orbital. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Atomic_orbital. License: CC BY-SA: Attribution-ShareAlike
- Sigma bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Sigma_bond. License: CC BY-SA: Attribution-ShareAlike
- sigma bond. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/sigma_bond. License: CC BY-SA: Attribution-ShareAlike
- covalent bond. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/covalent_bond. License: CC BY-SA: Attribution-ShareAlike
- atomic orbital. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/atomic_orbital. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- File:Covalent bond hydrogen.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Covalent_bond_hydrogen.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Sigma bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Sigma_bond.svg. License: CC BY-SA: Attribution-ShareAlike
- Neon orbitals. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Neon_orbitals.JPG. License: CC BY-SA: Attribution-ShareAlike
- Sigma-bonds-2D. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Sigma-bonds-2D.svg. License: CC BY-SA: Attribution-ShareAlike
- Triple bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Triple_bond. License: CC BY-SA: Attribution-ShareAlike
- Double bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Double_bond. License: CC BY-SA: Attribution-ShareAlike
- Orbital hybridisation. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Orbital_hybridisation. License: CC BY-SA: Attribution-ShareAlike
- atomic orbitals. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/atomic%20orbitals. License: CC BY-SA: Attribution-ShareAlike
- orbital hybridization. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/orbital%20hybridization. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- File:Covalent bond hydrogen.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Covalent_bond_hydrogen.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Sigma bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Sigma_bond.svg. License: CC BY-SA: Attribution-ShareAlike
- Neon orbitals. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Neon_orbitals.JPG. License: CC BY-SA: Attribution-ShareAlike
- Sigma-bonds-2D. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Sigma-bonds-2D.svg. License: CC BY-SA: Attribution-ShareAlike
- File:Sp3-Orbital.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Sp3-Orbital.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Pi-Bond. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Pi-Bond.svg. License: CC BY-SA: Attribution-ShareAlike
- Ethylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ethylene. License:
- Bond length. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Bond_length. License: CC BY-SA: Attribution-ShareAlike
- Bond dissociation energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Bond_dissociation_energy. License: CC BY-SA: Attribution-ShareAlike
- Covalent bonds. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Covalent_bonds. License: CC BY-SA: Attribution-ShareAlike
- Octet rule. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Octet_rule. License: CC BY-SA: Attribution-ShareAlike
- bond strength. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/bond%20strength. License: CC BY-SA: Attribution-ShareAlike
- octet rule. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/octet_rule. License: CC BY-SA: Attribution-ShareAlike
- NaF. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:NaF.gif. License: CC BY-SA: Attribution-ShareAlike
- File:Covalent bond hydrogen.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Covalent_bond_hydrogen.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Sigma bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Sigma_bond.svg. License: CC BY-SA: Attribution-ShareAlike
- Neon orbitals. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Neon_orbitals.JPG. License: CC BY-SA: Attribution-ShareAlike
- Sigma-bonds-2D. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Sigma-bonds-2D.svg. License: CC BY-SA: Attribution-ShareAlike
- File:Sp3-Orbital.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Sp3-Orbital.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Pi-Bond. Provided by: Wikimedia Commons. Located at: http://commons.wikimedia.org/wiki/File:Pi-Bond.svg. License: CC BY-SA: Attribution-ShareAlike
- Ethylene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ethylene. License:
This chapter is an adaptation of the chapter "The Covalent Bond" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
guideline that states main group atoms will form structures in which eight valence electrons interact with each nucleus, counting bonding electrons as interacting with both atoms connected by the bond
tendency of an atom to attract electrons in a bond to itself
A prefix used to name an aromatic ring with two substituents separated by one carbon on the ring.
distance between the nuclei of two bonded atoms at which the lowest potential energy is achieved

