59 Strengths of Ionic and Covalent Bonds
OpenStax
LEARNING OBJECTIVES
- Describe the energetics of covalent and ionic bond formation and breakage
- Use average covalent bond energies to estimate enthalpies of reaction
A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms.
It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process). In the case of [latex]\text{H}_2[/latex], the covalent bond is very strong; a large amount of energy, 436 kJ, must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate:
[latex]\text{H}_2 (g) \ \longrightarrow \ \text{2H} (g) \ \ \ \ \ \ \ \ \text{bond energy = 436 kJ}[/latex]
Conversely, the same amount of energy is released when one mole of [latex]\text{H}_2[/latex] molecules forms from two moles of H atoms:
[latex]\text{2H} (g) \ \longrightarrow \ \text{H}_2(g) \ \ \ \ \ \ \ \ \text{bond energy = -436 kJ}[/latex]
Bond Strength: Covalent Bonds
Stable molecules exist because covalent bonds hold the atoms together. We measure the strength of a covalent bond by the energy required to break it, that is, the energy necessary to separate the bonded atoms. Separating any pair of bonded atoms requires energy. The stronger a bond, the greater the energy required to break it.
The energy required to break a specific covalent bond in one mole of gaseous molecules is called the bond energy or the bond dissociation energy. The bond energy for a diatomic molecule, [latex]\text{D}_\text{X-Y}[/latex], is defined as the standard enthalpy change for the endothermic reaction:
[latex]\text{XY} (g) \longrightarrow \text{X} (g) \text{ + Y} (g) \ \ \ \ \ \text{D}_\text{X-Y} = \Delta H^{\circ}[/latex]
For example, the bond energy of the pure covalent H–H bond, [latex]\text{D}_\text{H-H}[/latex], is 436 kJ per mole of H–H bonds broken:
[latex]\text{H}_2 (g) \longrightarrow \text{2H} (g) \ \ \ \ \ \text{D}_\text{H-H} = \Delta H^{\circ} = \text{436 kJ}[/latex]
Molecules with three or more atoms have two or more bonds. The sum of all bond energies in such a molecule is equal to the standard enthalpy change for the endothermic reaction that breaks all the bonds in the molecule. For example, the sum of the four C–H bond energies in [latex]\text{CH}_4[/latex], 1660 kJ, is equal to the standard enthalpy change of the reaction: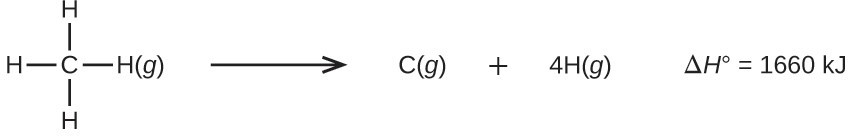
The average C–H bond energy, [latex]\text{D}_\text{C-H}[/latex], is 1660/4 = 415 kJ/mol because there are four moles of C–H bonds broken per mole of the reaction. Although the four C–H bonds are equivalent in the original molecule, they do not each require the same energy to break; once the first bond is broken (which requires 439 kJ/mol), the remaining bonds are easier to break. The 415 kJ/mol value is the average, not the exact value required to break any one bond.
The strength of a bond between two atoms increases as the number of electron pairs in the bond increases. Generally, as the bond strength increases, the bond length decreases. Thus, we find that triple bonds are stronger and shorter than double bonds between the same two atoms; likewise, double bonds are stronger and shorter than single bonds between the same two atoms. Average bond energies for some common bonds appear in the table below, and a comparison of bond lengths and bond strengths for some common bonds appears in the following table. When one atom bonds to various atoms in a group, the bond strength typically decreases as we move down the group. For example, [latex]\text{C–F}[/latex] is 439 kJ/mol, [latex]\text{C–Cl}[/latex] is 330 kJ/mol, and [latex]\text{C–Br}[/latex] is 275 kJ/mol.
| Bond Energies (kJ/mol) | |||||||
|---|---|---|---|---|---|---|---|
| Bond | Bond Energy | Bond | Bond Energy | Bond | Bond Energy | ||
| [latex]\text{H–H}[/latex] | 436 | [latex]\text{C–S}[/latex] | 260 | [latex]\text{F–Cl}[/latex] | 255 | ||
| [latex]\text{H–C}[/latex] | 415 | [latex]\text{C–Cl}[/latex] | 330 | [latex]\text{F–Br}[/latex] | 235 | ||
| [latex]\text{H–N}[/latex] | 390 | [latex]\text{C–Br}[/latex] | 275 | [latex]\text{Si–Si}[/latex] | 230 | ||
| [latex]\text{H–O}[/latex] | 464 | [latex]\text{C–I}[/latex] | 240 | [latex]\text{Si–P}[/latex] | 215 | ||
| [latex]\text{H–F}[/latex] | 569 | [latex]\text{N–N}[/latex] | 160 | [latex]\text{Si–S}[/latex] | 225 | ||
| [latex]\text{H–Si}[/latex] | 395 | [latex]\text{N=N}[/latex] | 418 | [latex]\text{Si–Cl}[/latex] | 359 | ||
| [latex]\text{H–P}[/latex] | 320 | [latex]\text{N} {\equiv} \text{N}[/latex] | 946 | [latex]\text{Si–Br}[/latex] | 290 | ||
| [latex]\text{H–S}[/latex] | 340 | [latex]\text{N–O}[/latex] | 200 | [latex]\text{Si–I}[/latex] | 215 | ||
| [latex]\text{H–Cl}[/latex] | 432 | [latex]\text{N–F}[/latex] | 270 | [latex]\text{P–P}[/latex] | 215 | ||
| [latex]\text{H–Br}[/latex] | 370 | [latex]\text{N–P}[/latex] | 210 | [latex]\text{P–S}[/latex] | 230 | ||
| [latex]\text{H–I}[/latex] | 295 | [latex]\text{N–Cl}[/latex] | 200 | [latex]\text{P–Cl}[/latex] | 330 | ||
| [latex]\text{C–C}[/latex] | 345 | [latex]\text{N–Br}[/latex] | 245 | [latex]\text{P–Br}[/latex] | 270 | ||
| [latex]\text{C=C}[/latex] | 611 | [latex]\text{O–O}[/latex] | 140 | [latex]\text{P–I}[/latex] | 215 | ||
| [latex]\text{C} {\equiv} \text{C}[/latex] | 837 | [latex]\text{O=O}[/latex] | 498 | [latex]\text{S–S}[/latex] | 215 | ||
| [latex]\text{C–N}[/latex] | 290 | [latex]\text{O–F}[/latex] | 160 | [latex]\text{S–Cl}[/latex] | 250 | ||
| [latex]\text{C=N}[/latex] | 615 | [latex]\text{O–Si}[/latex] | 370 | [latex]\text{S–Br}[/latex] | 215 | ||
| [latex]\text{C} {\equiv} \text{N}[/latex] | 891 | [latex]\text{O–P}[/latex] | 350 | [latex]\text{Cl–Cl}[/latex] | 243 | ||
| [latex]\text{C–O}[/latex] | 350 | [latex]\text{O–Cl}[/latex] | 205 | [latex]\text{Cl–Br}[/latex] | 220 | ||
| [latex]\text{C=O}[/latex] | 741 | [latex]\text{O–I}[/latex] | 200 | [latex]\text{Cl–I}[/latex] | 210 | ||
| [latex]\text{C} {\equiv} \text{O}[/latex] | 1080 | [latex]\text{F–F}[/latex] | 160 | [latex]\text{Br–Br}[/latex] | 190 | ||
| [latex]\text{C–F}[/latex] | 439 | [latex]\text{F–Si}[/latex] | 540 | [latex]\text{Br–I}[/latex] | 180 | ||
| [latex]\text{C–Si}[/latex] | 360 | [latex]\text{F–P}[/latex] | 489 | [latex]\text{I–I}[/latex] | 150 | ||
| [latex]\text{C–P}[/latex] | 265 | [latex]\text{F–S}[/latex] | 285 | ||||
| Average Bond Lengths and Bond Energies for Some Common Bonds | ||
|---|---|---|
| Bond | Bond Length (Å) | Bond Energy (kJ/mol) |
| [latex]\text{C–C}[/latex] | 1.54 | 345 |
| [latex]\text{C=C}[/latex] | 1.34 | 611 |
| [latex]\text{C} {\equiv} \text{C}[/latex] | 1.20 | 837 |
| [latex]\text{C–N}[/latex] | 1.43 | 290 |
| [latex]\text{C=N}[/latex] | 1.38 | 615 |
| [latex]\text{C} {\equiv} \text{N}[/latex] | 1.16 | 891 |
| [latex]\text{C–O}[/latex] | 1.43 | 350 |
| [latex]\text{C=O}[/latex] | 1.23 | 741 |
| [latex]\text{C} \equiv \text{O}[/latex] | 1.13 | 1080 |
The bond energy is the difference between the energy minimum (which occurs at the bond distance) and the energy of the two separated atoms. This is the quantity of energy released when the bond is formed. Conversely, the same amount of energy is required to break the bond. For the [latex]\text{H}_2[/latex] molecule shown in the table above, at the bond distance of 74 pm the system is 7.24 × 10−19 J lower in energy than the two separated hydrogen atoms. This may seem like a small number. However, as we will learn in more detail later, bond energies are often discussed on a per-mole basis. For example, it requires 7.24 × 10−19 J to break one H–H bond, but it takes 4.36 × 105 J to break 1 mole of H–H bonds. A comparison of some bond lengths and energies is shown in the tables above. We can find many of these bonds in a variety of molecules, and this table provides average values. For example, breaking the first C–H bond in [latex]\text{CH}_4[/latex] requires 439.3 kJ/mol, while breaking the first C–H bond in [latex]\text{H–CH}_2\text{C}_6\text{H}_5[/latex] (a common paint thinner) requires 375.5 kJ/mol.
As seen in the tables above, an average carbon-carbon single bond is 347 kJ/mol, while in a carbon-carbon double bond, the [latex]\pi[/latex] bond increases the bond strength by 267 kJ/mol. Adding an additional [latex]\pi[/latex] bond causes a further increase of 225 kJ/mol. We can see a similar pattern when we compare other [latex]\sigma[/latex] and [latex]\pi[/latex] bonds. Thus, each individual [latex]\pi[/latex] bond is generally weaker than a corresponding [latex]\sigma[/latex] bond between the same two atoms. In a [latex]\pi[/latex] bond, there is a greater degree of orbital overlap than in a [latex]\pi[/latex] bond.
We can use bond energies to calculate approximate enthalpy changes for reactions where enthalpies of formation are not available. Calculations of this type will also tell us whether a reaction is exothermic or endothermic. An exothermic reaction (ΔH negative, heat produced) results when the bonds in the products are stronger than the bonds in the reactants. An endothermic reaction (ΔH positive, heat absorbed) results when the bonds in the products are weaker than those in the reactants.
The enthalpy change, ΔH, for a chemical reaction is approximately equal to the sum of the energy required to break all bonds in the reactants (energy “in”, positive sign) plus the energy released when all bonds are formed in the products (energy “out,” negative sign). This can be expressed mathematically in the following way:
[latex]\Delta H= \Sigma \text{D}_\text{bonds broken}- \Sigma \text{D}_\text{bonds formed}[/latex]
In this expression, the symbol [latex]\Sigma[/latex] means “the sum of” and D represents the bond energy in kilojoules per mole, which is always a positive number. The bond energy is obtained from a table and will depend on whether the particular bond is a single, double, or triple bond. Thus, in calculating enthalpies in this manner, it is important that we consider the bonding in all reactants and products. Because D values are typically averages for one type of bond in many different molecules, this calculation provides a rough estimate, not an exact value, for the enthalpy of reaction.
Consider the following reaction:
[latex]\text{H}_2 (g) + \text{Cl}_2 (g) \longrightarrow \text{2HCl} (g)[/latex]
or
[latex]\text{H-H} (g) + \text{Cl-Cl} (g) \longrightarrow \text{2H-Cl} (g)[/latex]
To form two moles of [latex]\text{HCl}[/latex], one mole of H–H bonds and one mole of Cl–Cl bonds must be broken. The energy required to break these bonds is the sum of the bond energy of the H–H bond (436 kJ/mol) and the Cl–Cl bond (243 kJ/mol). During the reaction, two moles of H–Cl bonds are formed (bond energy = 432 kJ/mol), releasing 2 × 432 kJ; or 864 kJ. Because the bonds in the products are stronger than those in the reactants, the reaction releases more energy than it consumes:
| [latex]\Delta H[/latex] | [latex]=\Sigma \text{D}_\text{bonds broken} - \Sigma \text{D}_\text{bonds formed}[/latex] | |
| [latex]\Delta H[/latex] | [latex]= [\text{D}_\text{H-H} + \text{D}_\text{Cl-Cl}] - \text{2D}_\text{H-Cl}[/latex] | |
| [latex]=[436 + 243] - 2(432) = \text{-185 kJ}[/latex] |
This excess energy is released as heat, so the reaction is exothermic. Appendix G gives a value for the standard molar enthalpy of formation of [latex]\text{HCl} (g)[/latex], [latex]\Delta H_\text{f}^{\circ}[/latex], of –92.307 kJ/mol. Twice that value is –184.6 kJ, which agrees well with the answer obtained earlier for the formation of two moles of HCl.
EXAMPLE
Using Bond Energies to Calculate Approximate Enthalpy Changes
Methanol, [latex]\text{CH}_3\text{OH}[/latex], may be an excellent alternative fuel. The high-temperature reaction of steam and carbon produces a mixture of the gases carbon monoxide, [latex]\text{CO}[/latex], and hydrogen, [latex]\text{H}_2[/latex], from which methanol can be produced. Using the bond energies in the tables above, calculate the approximate enthalpy change, ΔH, for the reaction here:
[latex]\text{CO} (g) + \text{2H}_2 (g) \longrightarrow \text{CH}_3\text{OH} (g)[/latex]
Solution
First, we need to write the Lewis structures of the reactants and the products: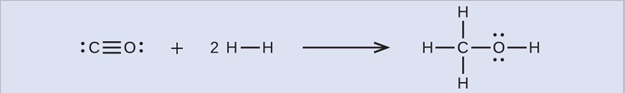
From this, we see that ΔH for this reaction involves the energy required to break a C–O triple bond and two H–H single bonds, as well as the energy produced by the formation of three C–H single bonds, a C–O single bond, and an O–H single bond. We can express this as follows:
| [latex]\Delta H =[/latex] | [latex]\Sigma \text{D}_\text{bonds broken} - \Sigma \text{D}_\text{bonds formed}[/latex] |
| [latex]\Delta H =[/latex] | [latex][\text{D}_{\text{C} {\equiv} \text{O}} + \text{2(D}_\text{H-H})] - [\text{3(D}_\text{C-H}) + \text{D}_\text{C-O} + \text{D}_\text{O-H}][/latex] |
Using the bond energy values in the table, we obtain:
| [latex]\Delta H[/latex] | [latex]= [1080 + 2(436)] - [3(415) + 350 + 464][/latex] |
| [latex]= \text{-107 kJ}[/latex] |
We can compare this value to the value calculated based on [latex]\Delta H_\text{f}^{\circ}[/latex] data from Appendix G:
| [latex]\Delta H[/latex] | [latex]= [\Delta H_\text{f}^{\circ} \text{CH}_3\text{OH} (g)] - [\Delta H_\text{f}^{\circ}\text{CO} (g) + 2 {\times} \Delta H_\text{f}^{\circ}\text{H}_2][/latex] |
| [latex]= [-201.0] - [-110.52 + 2 {\times} 0][/latex] | |
| [latex]= \text{-90.5 kJ}[/latex] |
Note that there is a fairly significant gap between the values calculated using the two different methods. This occurs because D values are the average of different bond strengths; therefore, they often give only rough agreement with other data.
Check Your Learning
Ethyl alcohol, [latex]\text{CH}_3\text{CH}_2\text{OH}[/latex], was one of the first organic chemicals deliberately synthesized by humans. It has many uses in industry, and it is the alcohol contained in alcoholic beverages. It can be obtained by the fermentation of sugar or synthesized by the hydration of ethylene in the following reaction:
Using the bond energies in the table, calculate an approximate enthalpy change, ΔH, for this reaction.
–35 kJ
Ionic Bond Strength and Lattice Energy
An ionic compound is stable because of the electrostatic attraction between its positive and negative ions. The lattice energy of a compound is a measure of the strength of this attraction. The lattice energy (ΔHlattice) of an ionic compound is defined as the energy required to separate one mole of the solid into its component gaseous ions. For the ionic solid MX, the lattice energy is the enthalpy change of the process:
[latex]\text{MX} (s) \longrightarrow \text{M}^{n+} (g) + \text{X}^{n-} (g) \ \ \ \ \Delta H_\text{lattice}[/latex]
Note that we are using the convention where the ionic solid is separated into ions, so our lattice energies will be endothermic (positive values). Some texts use the equivalent but opposite convention, defining lattice energy as the energy released when separate ions combine to form a lattice and giving negative (exothermic) values. Thus, if you are looking up lattice energies in another reference, be certain to check which definition is being used. In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. For sodium chloride, [latex]\Delta H_\text{lattice} = \text{769 kJ}[/latex]. Thus, it requires 769 kJ to separate one mole of solid [latex]\text{NaCl}[/latex] into gaseous [latex]\text{Na}^+[/latex] and [latex]\text{Cl}^-[/latex] ions. When one mole each of gaseous [latex]\text{Na}^+[/latex] and [latex]\text{Cl}^-[/latex] ions form solid [latex]\text{NaCl}[/latex], 769 kJ of heat is released.
The lattice energy [latex]\Delta H_\text{lattice}[/latex] of an ionic crystal can be expressed by the following equation (derived from Coulomb’s law, governing the forces between electric charges):
[latex]\Delta H_\text{lattice} = \frac{ \text{C(Z}^+\text{)(Z}^-) }{ \text{R}_\circ }[/latex]
in which C is a constant that depends on the type of crystal structure; Z+ and Z– are the charges on the ions; and Ro is the interionic distance (the sum of the radii of the positive and negative ions). Thus, the lattice energy of an ionic crystal increases rapidly as the charges of the ions increase and the sizes of the ions decrease. When all other parameters are kept constant, doubling the charge of both the cation and anion quadruples the lattice energy. For example, the lattice energy of [latex]\text{LiF}[/latex] (Z+ and Z– = 1) is 1023 kJ/mol, whereas that of [latex]\text{MgO}[/latex] (Z+ and Z– = 2) is 3900 kJ/mol (Ro is nearly the same—about 200 pm for both compounds).
Different interatomic distances produce different lattice energies. For example, we can compare the lattice energy of [latex]\text{MgF}_2[/latex] (2957 kJ/mol) to that of [latex]\text{MgI}_2[/latex] (2327 kJ/mol) to observe the effect on lattice energy of the smaller ionic size of F– as compared to I–.
EXAMPLE
Lattice Energy Comparisons
The precious gem ruby is aluminum oxide, [latex]\text{Al}_2\text{O}_3[/latex], containing traces of [latex]\text{Cr}^{3+}[/latex]. The compound [latex]\text{Al}_2\text{Se}_3[/latex] is used in the fabrication of some semiconductor devices. Which has the larger lattice energy, [latex]\text{Al}_2\text{O}_3[/latex] or [latex]\text{Al}_2\text{Se}_3[/latex]?
Solution
In these two ionic compounds, the charges Z+ and Z– are the same, so the difference in lattice energy will depend upon Ro. The [latex]\text{O}^{2-}[/latex] ion is smaller than the [latex]\text{Se}^{2-}[/latex] ion. Thus, [latex]\text{Al}_2\text{O}_3[/latex] would have a shorter interionic distance than [latex]\text{Al}_2\text{Se}_3[/latex], and [latex]\text{Al}_2\text{O}_3[/latex] would have the larger lattice energy.
Check Your Learning
Zinc oxide, [latex]\text{ZnO}[/latex], is a very effective sunscreen. How would the lattice energy of [latex]\text{ZnO}[/latex] compare to that of [latex]\text{NaCl}[/latex]?
[latex]\text{ZnO}[/latex] would have the larger lattice energy because the Z values of both the cation and the anion in [latex]\text{ZnO}[/latex] are greater, and the interionic distance of [latex]\text{ZnO}[/latex] is smaller than that of [latex]\text{NaCl}[/latex].
KEY TAKEAWAYS
The strength of a covalent bond is measured by its bond dissociation energy, that is, the amount of energy required to break that particular bond in a mole of molecules. Multiple bonds are stronger than single bonds between the same atoms. The enthalpy of a reaction can be estimated based on the energy input required to break bonds and the energy released when new bonds are formed. For ionic bonds, the lattice energy is the energy required to separate one mole of a compound into its gas phase ions. Lattice energy increases for ions with higher charges and shorter distances between ions.
- Bond energy for a diatomic molecule: [latex]\text{XY} (g) \longrightarrow \text{X} (g) + \text{Y} (g) \ \ \ \text{D}_\text{X-Y} = \Delta H^{\circ}[/latex]
- Enthalpy change: [latex]\Delta H = \Sigma \text{D}_\text{bonds broken} - \Sigma \text{D}_\text{bonds formed}[/latex]
- Lattice energy for a solid MX: [latex]\text{MX} (s) \longrightarrow \text{M}^{n+} (g) + \text{X}^{n-} (g) \Delta \ \ \ H_\text{lattice}[/latex]
- Lattice energy for an ionic crystal: [latex]\Delta H_\text{lattice} = \frac{ \text{C(Z}^+\text{)(Z}^-) }{ \text{R}_\circ }[/latex]
END OF CHAPTER EXERCISES
- Which bond in each of the following pairs of bonds is the strongest? (a) [latex]\text{C–C}[/latex] or [latex]\text{C=C}[/latex] (b) [latex]\text{C–N}[/latex] or [latex]\text{C} {\equiv} \text{N}[/latex] (c) [latex]\text{C} {\equiv} \text{O}[/latex] or [latex]\text{C=O}[/latex] (d) [latex]\text{H–F}[/latex] or [latex]\text{H–Cl}[/latex] (e) [latex]\text{C–H}[/latex] or [latex]\text{O–H}[/latex] (f) [latex]\text{C–N}[/latex] or [latex]\text{C–O}[/latex]
- Using the bond energies in the table, determine the approximate enthalpy change for each of the following reactions: (a) [latex]\text{H}_2 (g) + \text{Br}_2 (g) \longrightarrow \text{2HBr} (g)[/latex] (b) [latex]\text{CH}_4 (g) + \text{I}_2(g) \longrightarrow \text{CH}_3\text{I} (g) + \text{HI} (g)[/latex] (c) [latex]\text{C}_2\text{H}_4 (g) + \text{3O}_2 (g) \longrightarrow \text{2CO}_2 (g) + \text{2H}_2\text{O} (g)[/latex]
(a) −114 kJ; (b) 30 kJ; (c) −1055 kJ
- Using the bond energies in the table, determine the approximate enthalpy change for each of the following reactions: (a) [latex]\text{Cl}_2 (g) + \text{3F}_2 (g) \longrightarrow \text{2ClF}_3 (g)[/latex] (b) [latex]\text{H}_2\text{C} = \text{CH}_2 (g) + \text{H}_2 (g) \longrightarrow \text{H}_3\text{CCH}_3 (g)[/latex] (c) [latex]\text{2C}_2\text{H}_6 (g) + \text{7O}_2 (g) \longrightarrow \text{4CO}_2 (g) + \text{6H}_2\text{O} (g)[/latex]
- Draw a curve that describes the energy of a system with H and Cl atoms at varying distances. Then, find the minimum energy of this curve two ways.(a) Use the bond energy found in the tables to calculate the energy for one single [latex]\text{HCl}[/latex] bond (Hint: How many bonds are in a mole?)(b) Use the enthalpy of reaction and the bond energies for H2 and Cl2 to solve for the energy of one mole of [latex]\text{HCl}[/latex] bonds.
[latex]\text{H}_2 (g) + \text{Cl}_2 (g) \rightleftharpoons \text{2HCl} (g) \ \ \ \Delta H_\text{rxn}^{\circ} = \text{-184.7 kJ/mol}[/latex]
Explain why bonds occur at specific average bond distances instead of the atoms approaching each other infinitely close.
The specific average bond distance is the distance with the lowest energy. At distances less than the bond distance, the positive charges on the two nuclei repel each other, and the overall energy increases. - When a molecule can form two different structures, the structure with the stronger bonds is usually the more stable form. Use bond energies to predict the correct structure of the hydroxylamine molecule:
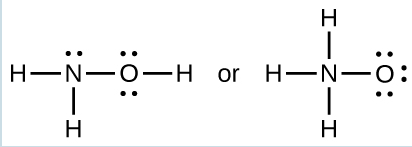 The greater bond energy is in the figure on the left. It is the more stable form.
The greater bond energy is in the figure on the left. It is the more stable form. - How does the bond energy of [latex]\text{HCl} (g)[/latex] differ from the standard enthalpy of formation of [latex]\text{HCl} (g)[/latex]?
- Using the standard enthalpy of formation data in Appendix G, show how the standard enthalpy of formation of [latex]\text{HCl} (g)[/latex] can be used to determine the bond energy.
[latex]\text{HCl} (g) \longrightarrow \frac{1}{2}\text{H}_2 (g) + \frac{1}{2}\text{Cl}_2 (g)[/latex] [latex]\Delta H_1^{\circ} = -\Delta H_{\text{f[HCl}(g)]}^{\circ}[/latex] [latex]\frac{1}{2}\text{H}_2 (g) \longrightarrow \text{H} (g)[/latex] [latex]\Delta H_2^{\circ} = \Delta H_{\text{f[H}(g)]}^{\circ}[/latex] [latex]\frac{1}{2}\text{Cl}_2 (g) \longrightarrow \text{Cl} (g)[/latex] [latex]\Delta H_3^{\circ} = \Delta H_{\text{f[Cl}(g)]}^{\circ}[/latex] [latex]\text{HCl} (g) \longrightarrow \text{H} (g) + \text{Cl} (g)[/latex] [latex]\Delta H_{298}^{\circ} = \Delta H_1^{\circ} + \Delta H_2^{\circ} + \Delta H_3^{\circ}[/latex] [latex]D_\text{HCl} = \Delta H_{298}^{\circ} = \Delta H_{\text{f[HCl}(g)]}^{\circ} + \Delta H_{\text{f[H}(g)]}^{\circ} + \Delta H_{\text{f[Cl}(g)]}^{\circ}[/latex] [latex]\text{= -(-92.307 kJ) + 217.97 kJ + 121.3 kJ}[/latex] [latex]\text{= 431.6 kJ}[/latex] - Using the standard enthalpy of formation data in Appendix G, calculate the bond energy of the carbon-sulfur double bond in [latex]\text{CS}_2[/latex].
- Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the S–F bond in [latex]\text{SF}_4 (g)[/latex] or in [latex]\text{SF}_6 (g)[/latex]?
The S–F bond in [latex]\text{SF}_4[/latex] is stronger.
- Using the standard enthalpy of formation data in Appendix G, determine which bond is stronger: the P–Cl bond in [latex]\text{PCl}_3 (g)[/latex] or in [latex]\text{PCl}_5 (g)[/latex]?
- Complete the following Lewis structure by adding bonds (not atoms), and then indicate the longest bond:

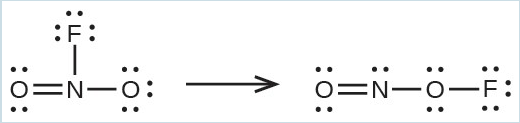
- Use the bond energy to calculate an approximate value of ΔH for the following reaction. Which is the more stable form of [latex]\text{FNO}_2[/latex]?
- Use principles of atomic structure to answer each of the following:1 (a) The radius of the Ca atom is 197 pm; the radius of the [latex]\text{Ca}^{2+}[/latex] ion is 99 pm. Account for the difference. (b) The lattice energy of [latex]\text{CaO} (s)[/latex] is –3460 kJ/mol; the lattice energy of [latex]\text{K}_2\text{O}[/latex] is –2240 kJ/mol. Account for the difference. (c) Given these ionization values, explain the difference between Ca and K with regard to their first and second ionization energies.
Element First Ionization Energy (kJ/mol) Second Ionization Energy (kJ/mol) K 419 3050 Ca 590 1140 (d) The first ionization energy of Mg is 738 kJ/mol and that of Al is 578 kJ/mol. Account for this difference.
(a) When two electrons are removed from the valence shell, the Ca radius loses the outermost energy level and reverts to the lower n = 3 level, which is much smaller in radius.(b) The +2 charge on calcium pulls the oxygen much closer compared with K, thereby increasing the lattice energy relative to a less charged ion.
(c) Removal of the 4s electron in Ca requires more energy than removal of the 4s electron in K because of the stronger attraction of the nucleus and the extra energy required to break the pairing of the electrons. The second ionization energy for K requires that an electron be removed from a lower energy level, where the attraction is much stronger from the nucleus for the electron. In addition, energy is required to unpair two electrons in a full orbital. For Ca, the second ionization potential requires removing only a lone electron in the exposed outer energy level.
(d) In Al, the removed electron is relatively unprotected and unpaired in a p orbital. The higher energy for Mg mainly reflects the unpairing of the 2s electron.
- For which of the following substances is the least energy required to convert one mole of the solid into separate ions? (a) [latex]\text{MgO}[/latex] (b) [latex]\text{SrO}[/latex] (c) [latex]\text{KF}[/latex] (d) [latex]\text{CsF}[/latex] (e) [latex]\text{MgF}_2[/latex]
(d)
- The reaction of a metal, [latex]\text{M}[/latex], with a halogen, [latex]\text{X}_2[/latex], proceeds by an exothermic reaction as indicated by this equation: [latex]\text{M} (s) + \text{X}_2 (g) \rightarrow \text{MX}_2 (s)[/latex]. For each of the following, indicate which option will make the reaction more exothermic. Explain your answers. (a) a large radius vs. a small radius for [latex]\text{M}^{+2}[/latex] (b) a high ionization energy vs. a low ionization energy for [latex]\text{M}[/latex] (c) an increasing bond energy for the halogen (d) a decreasing electron affinity for the halogen (e) an increasing size of the anion formed by the halogen
- The lattice energy of [latex]\text{LiF}[/latex] is 1023 kJ/mol, and the Li–F distance is 201 pm. [latex]\text{MgO}[/latex] crystallizes in the same structure as [latex]\text{LiF}[/latex] but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of [latex]\text{MgO}[/latex]: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4008 kJ/mol? Explain your choice.
4008 kJ/mol; both ions in [latex]\text{MgO}[/latex] have twice the charge of the ions in [latex]\text{LiF}[/latex]; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
- Which compound in each of the following pairs has the larger lattice energy? Note: [latex]\text{Mg}^{2+}[/latex] and [latex]\text{Li}^+[/latex] have similar radii; [latex]\text{O}^{2-}[/latex] and [latex]\text{F}^-[/latex] have similar radii. Explain your choices. (a) [latex]\text{MgO}[/latex] or [latex]\text{MgSe}[/latex] (b) [latex]\text{LiF}[/latex] or [latex]\text{MgO}[/latex] (c) [latex]\text{Li}_2\text{O}[/latex] or [latex]\text{LiCl}[/latex] (d) [latex]\text{Li}_2\text{Se}[/latex] or [latex]\text{MgO}[/latex]
- Which compound in each of the following pairs has the larger lattice energy? Note: [latex]\text{Ba}^{2+}[/latex] and [latex]\text{K}^+ [/latex] have similar radii; [latex]\text{S}^{2-}[/latex] and [latex]\text{Cl}^-[/latex] have similar radii. Explain your choices. (a) [latex]\text{K}_2\text{O}[/latex] or [latex]\text{Na}_2\text{O}[/latex] (b) [latex]\text{K}_2\text{S}[/latex] or [latex]\text{BaS}[/latex] (c) [latex]\text{KCl}[/latex] or [latex]\text{BaS}[/latex] (d) [latex]\text{BaS}[/latex] or [latex]\text{BaCl}_2[/latex]
(a) [latex]\text{Na}_2\text{O}[/latex]; [latex]\text{Na}^+[/latex] has a smaller radius than [latex]\text{K}^+[/latex]; (b) [latex]\text{BaS}[/latex]; [latex]\text{Ba}[/latex] has a larger charge than [latex]\text{K}[/latex]; (c) [latex]\text{BaS}[/latex]; [latex]\text{Ba}[/latex] and [latex]\text{S}[/latex] have larger charges; (d) [latex]\text{BaS}[/latex]; [latex]\text{S}[/latex] has a larger charge
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions? (a) [latex]\text{MgO}[/latex] (b) [latex]\text{SrO}[/latex] (c) [latex]\text{KF}[/latex] (d) [latex]\text{CsF}[/latex] (e) [latex]\text{MgF}_2[/latex]
- Which of the following compounds requires the most energy to convert one mole of the solid into separate ions? (a) [latex]\text{K}_2\text{S}[/latex] (b) [latex]\text{K}_2\text{O}[/latex] (c) [latex]\text{CaS}[/latex] (d) [latex]\text{Cs}_2\text{S}[/latex] (e) [latex]\text{CaO}[/latex]
(e)
Footnotes
- 1 This question is taken from the Chemistry Advanced Placement Examination and is used with the permission of the Educational Testing Service.
Glossary
- bond energy
- (also, bond dissociation energy) energy required to break a covalent bond in a gaseous substance
- lattice energy (ΔHlattice)
- energy required to separate one mole of an ionic solid into its component gaseous ions
This chapter is an adaptation of the chapter “Strengths of Ionic and Covalent Bonds” in Chemistry: Atoms First 2e by OpenStax and is licensed under a CC BY 4.0 license.
Access for free at https://openstax.org/books/chemistry-atoms-first-2e/pages/1-introduction
energy required to separate one mole of an ionic solid into its component gaseous ions