64 Strength of Acids
LumenLearning
Strong Acids
In water, strong acids completely dissociate into free protons and their conjugate base.
LEARNING OBJECTIVES
Calculate pH for solutions of strong acids.
KEY TAKEAWAYS
Key Points
- Strong acids can catalyze chemical reactions.
- Strong acids are defined by their [latex]\text{pK}_a[/latex]. The acid must be stronger in aqueous solution than a hydronium ion, so its [latex]\text{pK}_a[/latex] must be lower than that of a hydronium ion. Therefore, strong acids have a [latex]\text{pK}_a[/latex] of <-174.
- Strong acids can be organic or inorganic.
- Strong acids must be handled carefully because they can cause severe chemical burns.
- Strong acids are essential for catalyzing some reactions, including the synthesis and hydrolysis of carbonyl compounds.
Key Terms
- carbonyl: a divalent functional group ([latex]\text{-CO-}[/latex]), characteristic of aldehydes, ketones, carboxylic acids, amides, carboxylic acid anhydrides, carbonyl halides, esters, and others.
- ester: a compound usually formed by condensing an alcohol and an acid and eliminating of water. it contains the functional group carbon-oxygen double bond joined via carbon to another oxygen atom
- hydrolysis: a chemical process of decomposition; involves splitting a bond and adding the hydrogen cation and water’s hydroxide anion
Definition of Strong Acids
The strength of an acid refers to the ease with which the acid loses a proton. A strong acid ionizes completely in an aqueous solution by losing one proton, according to the following equation:
[latex]\text{HA} (aq) \rightarrow \text{H}^+ (aq) + \text{A}^- (aq)[/latex]
where [latex]\text{HA}[/latex] is a protonated acid, [latex]\text{H}^+[/latex] is the free acidic proton, and [latex]\text{A}^-[/latex] is the conjugate base. Strong acids yield weak conjugate bases. For sulfuric acid, which is diprotic, the “strong acid” designation refers only to the dissociation of the first proton:
[latex]\text{H}_2\text{SO}_4 (aq) \rightarrow \text{H}^+ (aq) + \text{HSO}_4^- (aq)[/latex]
More precisely, the acid must be stronger in aqueous solution than a hydronium ion ([latex]\text{H}^+[/latex]), so strong acids have a [latex]\text{pKa}[/latex] < -1.74. An example is hydrochloric acid ([latex]\text{HCl}[/latex]), whose [latex]\text{pKa}[/latex] is -6.3. This generally means that in aqueous solution at standard temperature and pressure, the concentration of hydronium ions is equal to the concentration of strong acid introduced to the solution.
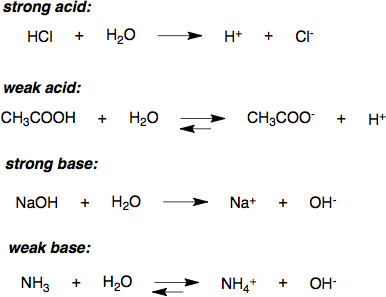
Due to the complete dissociation of strong acids in aqueous solution, the concentration of hydronium ions in the water is equal to the total concentration (ionized and un-ionized) of the acid introduced to solution:
[latex][ \text{H}^+ ] = [ \text{A}^- ] = [ \text{HA} ]_\text{total}[/latex] and [latex]\text{pH} = \text{-log}[ \text{H}^+ ][/latex].
Strong acids, like strong bases, can cause chemical burns when exposed to living tissue.
Examples of Strong Acids
Some common strong acids (acids with [latex]\text{pKa}[/latex] < -1) include:
- Hydroiodic acid ([latex]\text{HI}[/latex]): [latex]\text{pKa}[/latex] = -9.3
- Hydrobromic acid ([latex]\text{HBr}[/latex]): [latex]\text{pKa}[/latex] = -8.7
- Perchloric acid ([latex]\text{HClO}_4[/latex]): [latex]\text{pKa}[/latex] ≈ -8
- Hydrochloric acid ([latex]\text{HCl}[/latex]): [latex]\text{pKa}[/latex] = -6.3
- Sulfuric acid ([latex]\text{H}_2\text{SO}_4[/latex]): [latex]\text{pKa1}[/latex] ≈ -3 (first dissociation only)
- p-Toluenesulfonic acid: [latex]\text{pKa}[/latex] = -2.8
- Nitric acid ([latex]\text{HNO}_3[/latex]): [latex]\text{pKa}[/latex] ≈ -1.4
- Chloric acid ([latex]\text{HClO}_3[/latex]): [latex]\text{pKa}[/latex] ≈ 1.0
Weak Acids
A weak acid only partially dissociates in solution.
LEARNING OBJECTIVES
Solve acid-base equilibrium problems for weak acids.
KEY TAKEAWAYS
Key Points
- The dissociation of weak acids, which are the most common type of acid, are only partial.
Key Terms
- conjugate acid: the species created when a base accepts a proton
- conjugate base: the species created after donating a proton.
- weak acid: one that dissociates incompletely, donating only some of its hydrogen ions into solution
A weak acid is one that does not dissociate completely in solution; this means that a weak acid does not donate all of its hydrogen ions ([latex]\text{H}^+[/latex]) in a solution. Weak acids have very small values for [latex]\text{K}_a[/latex] (and therefore higher values for [latex]\text{pK}_a[/latex] ) compared to strong acids, which have very large [latex]\text{K}_a[/latex] values (and slightly negative [latex]\text{pK}_a[/latex] values).
The majority of acids are weak. On average, only about 1 percent of a weak acid solution dissociates in water in a 0.1 mol/L solution. Therefore, the concentration of [latex]\text{H}^+[/latex] ions in a weak acid solution is always less than the concentration of the undissociated species, [latex]\text{HA}[/latex]. Examples of weak acids include acetic acid ([latex]\text{CH}_a\text{COOH}[/latex]), which is found in vinegar, and oxalic acid ([latex]\text{H}_2\text{C}_2\text{O}_4[/latex]), which is found in some vegetables.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Acid strength. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Acid_strength%23Strong_acids. License: CC BY-SA: Attribution-ShareAlike
- Acid catalysis. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Acid_catalysis. License: CC BY-SA: Attribution-ShareAlike
- Strong and Weak Acids or Bases. Provided by: wilenskychemistry Wikispace. Located at: http://wilenskychemistry.wikispaces.com/Strong+and+Weak+Acids+or+Bases. License: CC BY-SA: Attribution-ShareAlike
- Acid strength. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Acid_strength%23Strong_acids. License: CC BY-SA: Attribution-ShareAlike
- Chemical burn. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_burn. License: CC BY-SA: Attribution-ShareAlike
- carbonyl. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/carbonyl. License: CC BY-SA: Attribution-ShareAlike
- ester. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ester. License: CC BY-SA: Attribution-ShareAlike
- hydrolysis. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/hydrolysis. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- A-level Chemistry/OCR (Salters)/Weak acids. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/A-level_Chemistry/OCR_(Salters)/Weak_acids. License: CC BY-SA: Attribution-ShareAlike
- Acid strength. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Acid_strength%23Weak_acids. License: CC BY-SA: Attribution-ShareAlike
- General Chemistry/Properties and Theories of Acids and Bases. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/General_Chemistry/Properties_and_Theories_of_Acids_and_Bases%23Strong_and_Weak_Acids.2FBases. License: CC BY-SA: Attribution-ShareAlike
- Weak acid. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Weak_acid%23Weak_acids. License: CC BY-SA: Attribution-ShareAlike
- conjugate base. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/conjugate_base. License: CC BY-SA: Attribution-ShareAlike
- conjugate acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/conjugate_acid. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- Essig-1. Provided by: Wikimedia. Located at: http://commons.wikimedia.org/wiki/File:Essig-1.jpg. License: CC BY-SA: Attribution-ShareAlike
- Common-ion effect. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Common-ion_effect. License: CC BY-SA: Attribution-ShareAlike
- Acid strength. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Acid_strength. License: CC BY-SA: Attribution-ShareAlike
- dissociation. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dissociation. License: CC BY-SA: Attribution-ShareAlike
- electrolyte. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/electrolyte. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: http://www.boundless.com//chemistry/definition/percent-ionization. License: CC BY-SA: Attribution-ShareAlike
- acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/acid. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- Original figure by Adrian Accurso. Licensed CC BY-SA 4.0. Provided by: Adrian Accurso. License: CC BY-SA: Attribution-ShareAlike
- Essig-1. Provided by: Wikimedia. Located at: http://commons.wikimedia.org/wiki/File:Essig-1.jpg. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter "Strength of Acids" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
a divalent functional group (-CO-), characteristic of aldehydes, ketones, carboxylic acids, amides, carboxylic acid anhydrides, carbonyl halides, esters, and others.
a compound usually formed by condensing an alcohol and an acid and eliminating of water. it contains the functional group carbon-oxygen double bond joined via carbon to another oxygen atom
a chemical process of decomposition; involves splitting a bond and adding the hydrogen cation and water’s hydroxide anion
one that dissociates incompletely, donating only some of its hydrogen ions into solution

