84 Proteins
LumenLearning
Amino Acids
An amino acid contains an amino group, a carboxyl group, and an R group, and it combines with other amino acids to form polypeptide chains.
LEARNING OBJECTIVES
Describe the structure of an amino acid and the features that confer its specific properties
KEY TAKEAWAYS
Key Points
- Each amino acid contains a central C atom, an amino group ([latex]\text{NH}_2[/latex]), a carboxyl group ([latex]\text{COOH}[/latex]), and a specific R group.
- The R group determines the characteristics (size, polarity, and pH) for each type of amino acid.
- Peptide bonds form between the carboxyl group of one amino acid and the amino group of another through dehydration synthesis.
- A chain of amino acids is a polypeptide.
Key Terms
- amino acid: Any of 20 naturally occurring α-amino acids (having the amino, and carboxylic acid groups on the same carbon atom), and a variety of side chains, that combine, via peptide bonds, to form proteins.
- R group: The R group is a side chain specific to each amino acid that confers particular chemical properties to that amino acid.
- polypeptide: Any polymer of (same or different) amino acids joined via peptide bonds.
Structure of an Amino Acid
Amino acids are the monomers that make up proteins. Each amino acid has the same fundamental structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group ([latex]\text{NH}_2[/latex]), a carboxyl group ([latex]\text{COOH}[/latex]), and to a hydrogen atom. In the aqueous environment of the cell, the both the amino group and the carboxyl group are ionized under physiological conditions, and so have the structures [latex]\text{-NH}_3^+[/latex] and [latex]\text{-COO}^-[/latex], respectively. Every amino acid also has another atom or group of atoms bonded to the central atom known as the R group. This R group, or side chain, gives each amino acid proteins specific characteristics, including size, polarity, and pH.
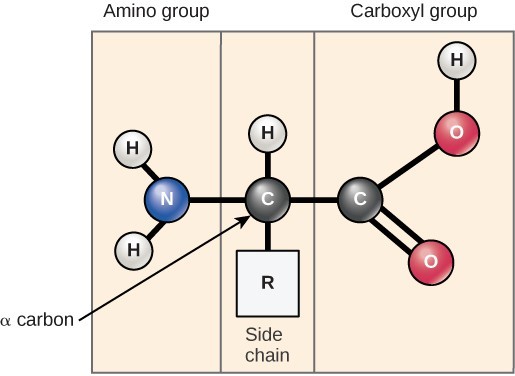
Types of Amino Acids
The name “amino acid” is derived from the amino group and carboxyl-acid-group in their basic structure. There are 21 amino acids present in proteins, each with a specific R group or side chain. Ten of these are considered essential amino acids in humans because the human body cannot produce them and they must be obtained from the diet. All organisms have different essential amino acids based on their physiology.
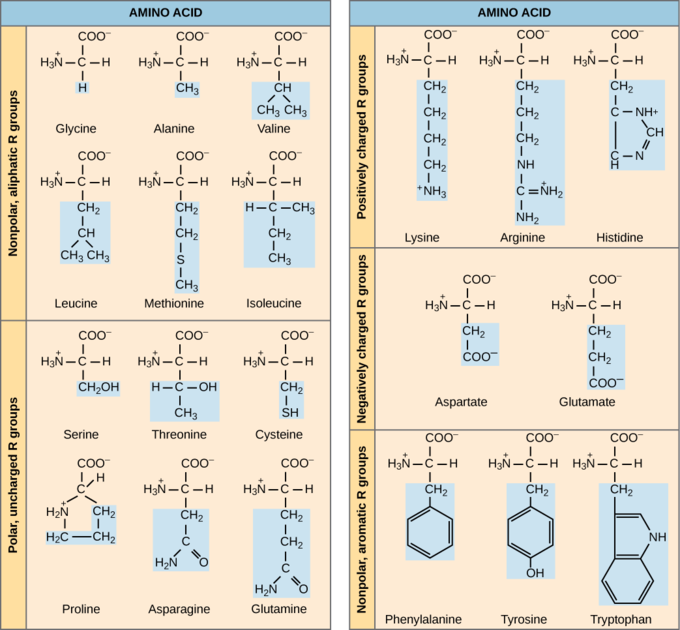
Characteristics of Amino Acids
Which categories of amino acid would you expect to find on the surface of a soluble protein, and which would you expect to find in the interior? What distribution of amino acids would you expect to find in a protein embedded in a lipid bilayer?
The chemical composition of the side chain determines the characteristics of the amino acid. Amino acids such as valine, methionine, and alanine are nonpolar (hydrophobic), while amino acids such as serine, threonine, and cysteine are polar (hydrophilic). The side chains of lysine and arginine are positively charged so these amino acids are also known as basic (high pH) amino acids. Proline is an exception to the standard structure of an amino acid because its R group is linked to the amino group, forming a ring-like structure.
Amino acids are represented by a single upper case letter or a three-letter abbreviation. For example, valine is known by the letter V or the three-letter symbol val.
Peptide Bonds
The sequence and the number of amino acids ultimately determine the protein’s shape, size, and function. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond. When two amino acids are covalently attached by a peptide bond, the carboxyl group of one amino acid and the amino group of the incoming amino acid combine and release a molecule of water. Any reaction that combines two monomers in a reaction that generates [latex]\text{H}_2\text{O}[/latex] as one of the products is known as a dehydration reaction, so peptide bond formation is an example of a dehydration reaction.
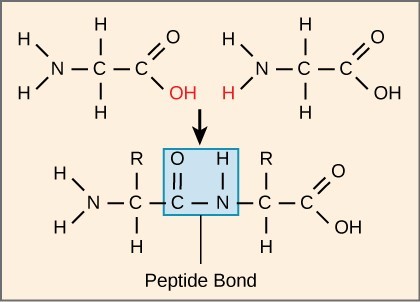
Polypeptide Chains
The resulting chain of amino acids is called a polypeptide chain. Each polypeptide has a free amino group at one end. This end is called the N terminal, or the amino terminal, and the other end has a free carboxyl group, also known as the C or carboxyl terminal. When reading or reporting the amino acid sequence of a protein or polypeptide, the convention is to use the N-to-C direction. That is, the first amino acid in the sequence is assumed to the be one at the N terminal and the last amino acid is assumed to be the one at the C terminal.
Although the terms polypeptide and protein are sometimes used interchangeably, a polypeptide is technically any polymer of amino acids, whereas the term protein is used for a polypeptide or polypeptides that have folded properly, combined with any additional components needed for proper functioning, and is now functional.
Peptide Bonding between Amino Acids
The peptide bond is an amide bond which links amino acids together to form proteins.
LEARNING OBJECTIVES
Identify the amino acids that were combined to create a peptide.
KEY TAKEAWAYS
Key Points
- An amide bond has various resonance forms which allow for extra stabilization and extra versatility in various environments.
- Amino acids is the basic building block of proteins; they are composed of a carbon atom attached to a hydrogen, a carbonyl group, an amine group, and an R group. Large proteins are formed by linking amino acids with peptide bonds.
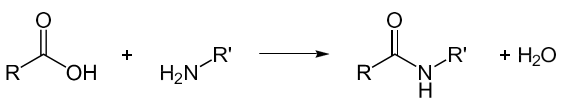
- The amide bond is formed through a condensation reaction, whereby the carbonyl and the amine group link together with the release of water.
Key Terms
- amino acid: any of the 20 naturally occurring α-amino acids (having the amino and carboxylic acid groups on the same carbon atom) and a variety of side chains that combine, via peptide bonds, to form proteins
- dipole: any molecule or radical that has delocalized positive and negative charges
Amino acids are the building blocks for the proteins responsible for the biological functions within our body. Amino acids are chemical compounds consisting of a carbon atom bonded to an amine group, a hydrogen atom, a carboxylic group, and a varying side-chain (R group); it is this side chain that distinguishes each amino acid from another. Higher-ordered structures such as peptide chains and proteins are formed when amino acids bond to each other.
Peptides
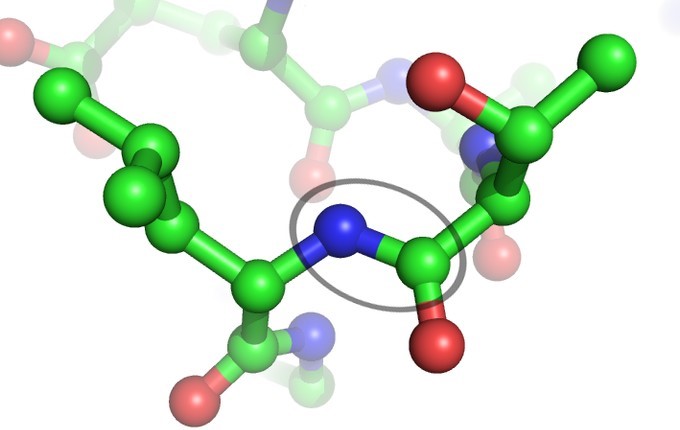
A peptides is a molecule composed of two or more amino acids. The bond that holds together the two amino acids is a peptide bond, or a covalent chemical bond between two compounds (in this case, two amino acids). It occurs when the carboxylic group of one molecule reacts with the amino group of the other molecule, linking the two molecules and releasing a water molecule.
Long chain polypeptides can be formed by linking many amino acids to each other via peptide bonds. The amide bond can only be broken by amide hydrolysis, where the bonds are cleaved with the addition of a water molecule. The peptide bonds of proteins are metastable, and will break spontaneously in a slow process. Living organisms have enzymes which are capable of both forming and breaking peptide bonds.
Resonance Forms of the Amid Group
The amide group has three resonance forms, which confer important properties. First, the stabilization afforded from the resonance structures effectively stabilizes it by 80kj/mol, making it less reactive than similar groups. The peptide bond is uncharged at normal pH values, but the double bonded character from the resonance structure creates a dipole, which can line up in secondary structures. The partial double bond character can be strengthened or weakened by modifications that favor one another, allowing some flexibility for the presence of the peptide group in varying conditions. The extra stabilization makes the peptide bond relatively stable and unreactive. However, peptide bonds can undergo chemical reactions, typically through an attack of the electronegative atom on the carbonyl carbon, resulting in the formation of a tetrahedral intermediate.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- amino acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/amino_acid. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/?collection=col11448/latest. License: CC BY: Attribution
- Boundless. Provided by: Boundless Learning. Located at: http://www.boundless.com//biology/definition/r-group. License: CC BY-SA: Attribution-ShareAlike
- polypeptide. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/polypeptide. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_03.jpg. License: CC BY: Attribution
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_02.png. License: CC BY: Attribution
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_01.jpg. License: CC BY: Attribution
- amino acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/amino_acid. License: CC BY-SA: Attribution-ShareAlike
- Peptide bonds. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Peptide_bonds. License: CC BY-SA: Attribution-ShareAlike
- dipole. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dipole. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_03.jpg. License: CC BY: Attribution
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_02.png. License: CC BY: Attribution
- OpenStax College, Proteins. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44402/latest/Figure_03_04_01.jpg. License: CC BY: Attribution
- Peptide bond. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Peptide_bond.png. License: CC BY-SA: Attribution-ShareAlike
- File:Amidbildung.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Amidbildung.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter "Proteins" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.

