52 Solubility
LumenLearning
Solubility is the relative ability of a solute (solid, liquid, or gas) to dissolve into a solvent and form a solution.
LEARNING OBJECTIVES
Recognize the various ions that cause a salt to generally be soluble/insoluble in water.
KEY TAKEAWAYS
Key Points
- Solubility is the relative ability of a solute to dissolve into a solvent.
- Several factors affect the solubility of a given solute in a given solvent. Temperature often plays the largest role, although pressure can have a significant effect for gases.
- To predict whether a compound will be soluble in a given solvent, remember the saying, “Like dissolves like.” Highly polar ionic compounds such as salt readily dissolve in polar water, but do not readily dissolve in non-polar solutions such as benzene or chloroform.
Key Terms
Definition of Solubility
Solubility is the ability of a solid, liquid, or gaseous chemical substance (referred to as the solute) to dissolve in solvent (usually a liquid) and form a solution. The solubility of a substance fundamentally depends on the solvent used, as well as temperature and pressure. The solubility of a substance in a particular solvent is measured by the concentration of the saturated solution. A solution is considered saturated when adding additional solute no longer increases the concentration of the solution.
The degree of solubility ranges widely depending on the substances, from infinitely soluble (fully miscible), such as ethanol in water, to poorly soluble, such as silver chloride in water. The term “insoluble” is often applied to poorly soluble compounds. Under certain conditions, the equilibrium solubility can be exceeded, yielding a supersaturated solution.
Solubility does not depend on particle size; given enough time, even large particles will eventually dissolve.
Factors Affecting Solubility
Temperature
The solubility of a given solute in a given solvent typically depends on temperature. For many solids dissolved in liquid water, solubility tends to correspond with increasing temperature. As water molecules heat up, they vibrate more quickly and are better able to interact with and break apart the solute.
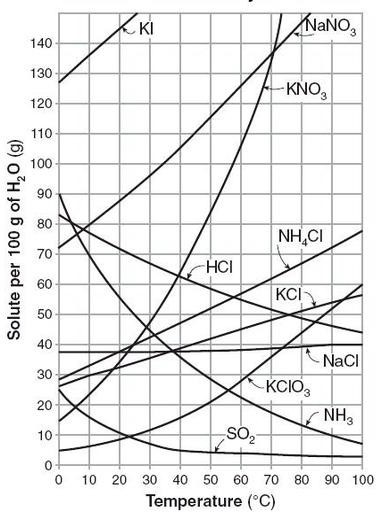
The solubility of gases displays the opposite relationship with temperature; that is, as temperature increases, gas solubility tends to decrease. In a chart of solubility vs. temperature, notice how solubility tends to increase with increasing temperature for the salts and decrease with increasing temperature for the gases.
Pressure
Pressure has a negligible effect on the solubility of solid and liquid solutes, but it has a strong effect on solutions with gaseous solutes. This is apparent every time you open a soda can; the hissing sound from the can is due to the fact that its contents are under pressure, which ensures that the soda stays carbonated (that is to say, that the carbon dioxide stays dissolved in solution). The takeaway from this is that the solubility of gases tends to correlate with increasing pressure.
Polarity
A popular saying used for predicting solubility is “Like dissolves like.” This statement indicates that a solute will dissolve best in a solvent that has a similar chemical structure; the ability for a solvent to dissolve various compounds depends primarily on its polarity. For example, a polar solute such as sugar is very soluble in polar water, less soluble in moderately polar methanol, and practically insoluble in non-polar solvents such as benzene. In contrast, a non-polar solute such as naphthalene is insoluble in water, moderately soluble in methanol, and highly soluble in benzene.
Solubility Chart
The solubility chart shows the solubility of many salts. Salts of alkali metals (and ammonium), as well as those of nitrate and acetate, are always soluble. Carbonates, hydroxides, sulfates, phosphates, and heavy metal salts are often insoluble.
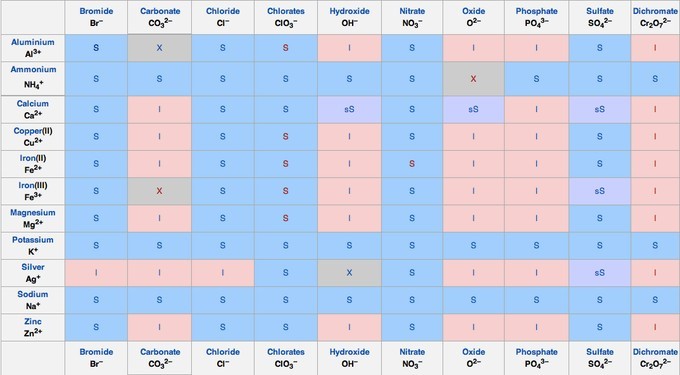
Solubility: Solubility of salt and gas solutes in liquid solvent.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- General Chemistry/Solubility. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/General_Chemistry/Solubility. License: CC BY-SA: Attribution-ShareAlike
- Precipitation (chemistry). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Precipitation_(chemistry). License: CC BY-SA: Attribution-ShareAlike
- General Chemistry/Properties of Solutions. Provided by: Wikibooks. Located at: http://en.wikibooks.org/wiki/General_Chemistry/Properties_of_Solutions. License: CC BY-SA: Attribution-ShareAlike
- precipitation. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/precipitation. License: CC BY-SA: Attribution-ShareAlike
- solution. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/solution. License: CC BY-SA: Attribution-ShareAlike
- precipitation reaction. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/precipitation_reaction. License: CC BY-SA: Attribution-ShareAlike
- Solubility chart. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Solubility_chart. License: Public Domain: No Known Copyright
- Solubility. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Solubility. License: CC BY-SA: Attribution-ShareAlike
- miscible. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/miscible. License: CC BY-SA: Attribution-ShareAlike
- Solubility chart. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Solubility_chart. License: Public Domain: No Known Copyright
- Solubilty of various substances vs. temperature change. Provided by: Wikispaces. Located at: http://chem409-fouling.wikispaces.com/Fouling+Mechanisms. License: CC BY-SA: Attribution-ShareAlike
- Solubility. Located at: http://www.youtube.com/watch?v=zjIVJh4JLNo. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Boundless. Provided by: Boundless Learning. Located at: https://figures.boundless.com/9231/large/solubility-20chart.png. License: CC BY-SA: Attribution-ShareAlike
- Ionic equation. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ionic_equation. License: CC BY-SA: Attribution-ShareAlike
- Spectator ion. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Spectator_ion. License: CC BY-SA: Attribution-ShareAlike
- electrolyte. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/electrolyte. License: CC BY-SA: Attribution-ShareAlike
- salt. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/salt. License: CC BY-SA: Attribution-ShareAlike
- Solubility chart. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Solubility_chart. License: Public Domain: No Known Copyright
- Solubilty of various substances vs. temperature change. Provided by: Wikispaces. Located at: http://chem409-fouling.wikispaces.com/Fouling+Mechanisms. License: CC BY-SA: Attribution-ShareAlike
- Solubility. Located at: http://www.youtube.com/watch?v=zjIVJh4JLNo. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Boundless. Provided by: Boundless Learning. Located at: https://figures.boundless.com/9231/large/solubility-20chart.png. License: CC BY-SA: Attribution-ShareAlike
- Chlorid stu0159brnu00fd. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Chlorid_st%C5%99%C3%ADbrn%C3%BD.PNG. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter “Precipitation Reactions” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
solution component present in a concentration less than that of the solvent
solution component present in a concentration that is higher relative to other components

