79 Functional Groups Names, Properties, and Reactions
LumenLearning
Functional Groups
Functional groups refer to specific atoms bonded in a certain arrangement that give a compound certain physical and chemical properties.
LEARNING OBJECTIVES
Define the term “functional group” as it applies to organic molecules
KEY TAKEAWAYS
Key Points
- Functional groups are often used to “functionalize” a compound, affording it different physical and chemical properties than it would have in its original form.
- Functional groups will undergo the same type of reactions regardless of the compound of which they are a part; however, the presence of certain functional groups within close proximity can limit reactivity.
- Functional groups can be used to distinguish similar compounds from each other.
Key Terms
- functional group: A specific grouping of elements that is characteristic of a class of compounds, and determines some properties and reactions of that class.
- functionalization: Addition of specific functional groups to afford the compound new, desirable properties.
The Role of Functional Groups
In organic chemistry, a functional group is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound. The same functional group will behave in a similar fashion, by undergoing similar reactions, regardless of the compound of which it is a part. Functional groups also play an important part in organic compound nomenclature; combining the names of the functional groups with the names of the parent alkanes provides a way to distinguish compounds.
The atoms of a functional group are linked together and to the rest of the compound by covalent bonds. The first carbon atom that attaches to the functional group is referred to as the alpha carbon; the second, the beta carbon; the third, the gamma carbon, etc. Similarly, a functional group can be referred to as primary, secondary, or tertiary, depending on if it is attached to one, two, or three carbon atoms.
Functional Groups and Reactivity
Functional groups play a significant role in directing and controlling organic reactions. Alkyl chains are often nonreactive, and the direction of site-specific reactions is difficult; unsaturated alkyl chains with the presence of functional groups allow for higher reactivity and specificity. Often, compounds are functionalized with specific groups for a specific chemical reaction. Functionalization refers to the addition of functional groups to a compound by chemical synthesis. Through routine synthesis methods, any kind of organic compound can be attached to the surface. In materials science, functionalization is employed to achieve desired surface properties; functional groups can also be used to covalently link functional molecules to the surfaces of chemical devices.
In organic chemistry, the most common functional groups are carbonyls ([latex]\text{C=O}[/latex]), alcohols ([latex]\text{-OH}[/latex]), carboxylic acids ([latex]\text{CO}_2\text{H}[/latex]), esters ([latex]\text{CO}_2\text{R}[/latex]), and amines ([latex]\text{NH}_2[/latex]). It is important to be able to recognize the functional groups and the physical and chemical properties that they afford compounds.
Organic chemistry functional groups lesson: This video provides a great overview of the various functional groups in organic chemistry.
Alcohols
Alcohols are functional groups characterized by the presence of an [latex]\text{-OH}[/latex] group.
LEARNING OBJECTIVES
Identify the general properties of the alcohol functional group
KEY TAKEAWAYS
Key Points
- Due to the presence of an [latex]\text{-OH}[/latex] group, alcohols can hydrogen bond. This leads to higher boiling points compared to their parent alkanes.
- Alcohols are polar in nature. This is attributed to the difference in electronegativity between the carbon and the oxygen atoms.
- In chemical reactions, alcohols often cannot leave the molecule on their own; to leave, they often become protonated to water, which is a better leaving group. Alcohols also can become deprotonated in the presence of a strong base.
Key Terms
- alkane: Any of the saturated hydrocarbons—including methane, ethane, and compounds with long carbon chain known as paraffins, etc.— that have a chemical formula of the form [latex]\text{C}_\text{n}\text{H}_{2n+2}[/latex].
- aldehyde: Any of a large class of reactive organic compounds ([latex]\text{RCHO}[/latex]) having a carbonyl functional group attached to one hydrocarbon radical and a hydrogen atom.
- carboxylic acid: Any of a class of organic compounds containing a carboxyl functional group—a carbon with a double bond to an oxygen and a single bond to another oxygen, which is in turn bonded to a hydrogen.
- leaving group: In organic chemistry, the species that leaves the parent molecule following a substitution reaction.
Alcohols are organic compounds in which the hydroxyl functional group ([latex]\text{-OH}[/latex]) is bound to a carbon atom. Alcohols are an important class of molecules with many scientific, medical, and industrial uses.
Nomenclature of Alcohols
According to the IUPAC nomenclature system, an alcohol is named by dropping the terminal “-e” of the parent carbon chain (alkane, alkene, or alkyne in most cases) and the addition of “-ol” as the ending. If the location of the hydroxyl group must be specified, a number is inserted between the parent alkane name and the “-ol” (propan-1-ol) or before the IUPAC name (1-propanol). If a higher priority group is present, such as an aldehyde, ketone or carboxylic acid, then it is necessary to use the prefix “hydroxy-” instead of the ending “-ol.”
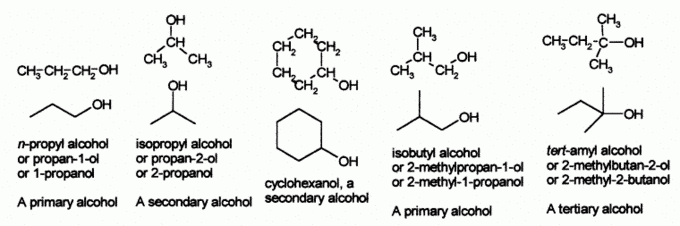
Alcohols are classified as primary, secondary, or tertiary, based upon the number of carbon atoms connected to the carbon atom that bears the hydroxyl group.
Structure and Physical Properties of Alcohols
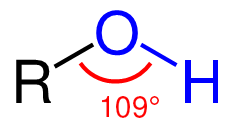
The structure of an alcohol is similar to that of water, as it has a bent shape. This geometrical arrangement reflects the effect of electron repulsion and the increasing steric bulk of the substituents on the central oxygen atom. Like water, alcohols are polar, containing an unsymmetrical distribution of charge between the oxygen and hydrogen atoms. The high electronegativity of the oxygen compared to carbon leads to the shortening and strengthening of the [latex]\text{-OH}[/latex] bond. The presence of the [latex]\text{-OH}[/latex] groups allows for hydrogen bonding with other [latex]\text{-OH}[/latex] groups, hydrogen atoms, and other molecules. Since alcohols are able to hydrogen bond, their boiling points are higher than those of their parent molecules.
Alcohols are able to participate in many chemical reactions. They often undergo deprotonation in the presence of a strong base. This weak acid behavior results in the formation in an alkoxide salt and a water molecule. Hydroxyl groups alone are not considered good leaving groups. Often, their participation in nucleophilic substitution reactions is instigated by the protonation of the oxygen atom, leading to the formation a water moiety—a better leaving group. Alcohols can react with carboxylic acids to form an ester, and they can be oxidized to aldehydes or carboxylic acids.
Alcohols have many uses in our everyday world. They are found in beverages, antifreeze, antiseptics, and fuels. They can be used as preservatives for specimens in science, and they can be used in industry as reagents and solvents because they display an ability to dissolve both polar and non-polar substances.
Ethers
Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups.
LEARNING OBJECTIVES
Define the term “ether” as it relates to organic compounds
KEY TAKEAWAYS
Key Points
- Ethers have relatively low boiling points due to their inability to form hydrogen bonds with each other.
- Due to the electronegativity difference between the oxygen and carbon atoms of an ether, the molecule is slightly polar.
- Although they have low reactivity overall, the two lone pairs of electrons on the oxygen atom do afford the ether molecule some reactivity; the ether molecule is subject to reacting with strong acids and serves as a Lewis base.
Key Terms
- alkene: An unsaturated, aliphatic hydrocarbon with one or more carbon–carbon double bond.
- ester: A compound most often formed by the condensation of an alcohol and an acid, with elimination of water. It contains the functional group [latex]\text{C=O}[/latex] joined via carbon to another oxygen atom.
- ether: Compound containing an oxygen atom bonded to two hydrocarbon groups.
- amide: Any derivative of an oxoacid in which the hydroxyl group has been replaced with an amino or substituted amino group; especially such derivatives of a carboxylic acid, the carboxamides.
Structure of Ethers
Ethers are a class of organic compounds that contain an ether group. An ether group is an oxygen atom connected to two alkyl or aryl groups. They follow the general formula [latex]\text{R-O-R’}[/latex]. The [latex]\text{C-O-C}[/latex] linkage is characterized by bond angles of 104.5 degrees, with the [latex]\text{C-O}[/latex] distances being about 140 pm. The oxygen of the ether is more electronegative than the carbons. Thus, the alpha hydrogens are more acidic than in regular hydrocarbon chains.
Nomenclature of Ethers
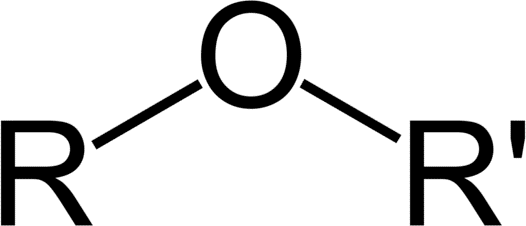
There are two ways to name ethers. The most common way is to identify the alkyl groups on either side of the oxygen atom in alphabetical order, then write “ether.” For example, ethyl methyl ether is the ether that has an ethyl group and a methyl group on either side of the oxygen atom. If the two alkyl groups are identical, the ether is called di[alkyl] ether. For example, diethyl ether is the ether with an ethyl group on each side of the oxygen atom.
The other way of naming ethers is the formal, IUPAC method. This way, the form is: [short alkyl chain][oxy][long alkyl chain]. For example, the IUPAC name for ethyl methyl ether would be methoxyethane.
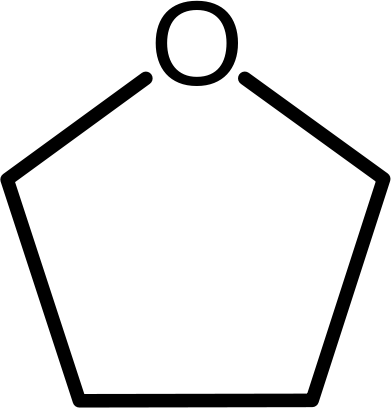
In cyclic ethers, the stem of the compound is known as a oxacycloalkane. The “oxa” is an indicator of the replacement of the carbon by an oxygen in the ring. An example is oxacyclopentane, a five-membered ring in which there are four carbon atoms and one oxygen atom.
Properties of Ethers
Ethers are rather nonpolar due to the presence of an alkyl group on either side of the central oxygen. The presence of the bulky alkyl groups that are adjacent to it means that the oxygen atom is largely unable to participate in hydrogen bonding. Ethers, therefore, have lower boiling points compared to alcohols of similar molecular weight. However, as the alkyl chain of the ethers becomes longer, the difference in boiling points becomes smaller. This is due to the effect of increased Van der Waals interactions as the number of carbons increases, and therefore the number of electrons increases as well. The two lone pairs of electrons present on the oxygen atoms make it possible for ethers to form hydrogen bonds with water. Ethers are more polar than alkenes, but not as polar as esters, alcohols or amides of comparable structures.
Reactions
Ethers have relatively low chemical reactivity, but they are still more reactive than alkanes. Although they resist undergoing hydrolysis, they are often cleaved by acids, which results in the formation of an alkyl halide and an alcohol. Ethers tend to form peroxides in the presence of oxygen or air. The general formula is [latex]\text{R-O-O-R’}[/latex]. Ethers can serve as Lewis and Bronsted bases, serving to donate electrons in reactions, or accept protons. Ethers can be formed in the laboratory through the dehydration of alcohols ([latex]\text{2R-OH} \rightarrow \text{R-O-R + H}_2\text{O}[/latex] at high temperature), nucleophilic displacement of alkyl halides by alkoxides ([latex]\text{R-ONa + R’-X} \rightarrow \text{R-O-R’ + NaX}[/latex]), or electrophilic addition of alcohols to alkenes ([latex]\text{R}_2\text{C=CR}_2 \text{+ R-OH} \rightarrow \text{R}_2\text{CH-C(-O-R)-R}_2[/latex]).
Aldehydes and Ketones
Aldehydes and ketones are classes of organic compounds that contain a carbonyl ([latex]\text{C=O}[/latex]) group.
LEARNING OBJECTIVES
Identify the general properties of ketones and aldehydes
KEY TAKEAWAYS
Key Points
- The carbonyl functional group is a carbon double bonded to an oxygen. Depending on the location of the carbonyl group, it is termed differently; ketones contain the carbonyl inside the compound and aldehydes contain the carbonyl at the end of the organic compound.
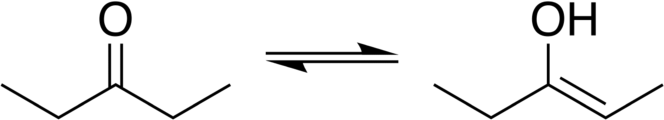
- Ketones and aldehydes can undergo keto- enol tautomerism. This refers to the equilibrium between the two possible tautomers. The interconversion of the two forms involves the movement of a proton and the shifting of bonding electrons. This equilibrium affords the compounds more reactivity.
- Ketones and aldehydes participate in a variety of reactions. They can undergo oxidation reactions, in which they become oxidized to the corresponding carboxylic acids.
Key Terms
- tautomerism: A form of isomerism in which a dynamic equilibrium between multiple isomers exists, such as that between an enol and a ketone.
- oxidize: To increase the valence (the positive charge) of an element by removing electrons.
- aldehyde: An organic compound containing a formyl group, which is a functional group with the structure [latex]\text{R-CHO}[/latex].
- sp2: Hybrid orbital that forms when one pi bond is required for the double bond, and only three σ bonds are formed per carbon atom. The 2s orbital is mixed with only two of the three 2p orbitals.
- ketone: A compound containing an oxygen atom joined to a carbon atom by a double bond.
In organic chemistry, a carbonyl group is a functional group which has a carbon double bonded to an oxygen atom: [latex]\text{C=O}[/latex].
Ketones
When a carbonyl functional group is placed within a molecule, it is known as a ketone. Ketones are organic compounds with the structure [latex]\text{RC(=O)R’}[/latex], where [latex]\text{R}[/latex] and [latex]\text{R’}[/latex] can be a variety of carbon-containing substituents. IUPAC nomenclature rules dictate that ketone molecules are named by changing the suffix of the parent carbon molecule to “-one.” If the position of the ketone must be specified, then a number is placed between the parent chain name and the “-one” prefix (e.g., propan-2-one), or at the beginning of the IUPAC name. The prefixes “oxo-” and “keto-” are used to describe the ketone functional group.
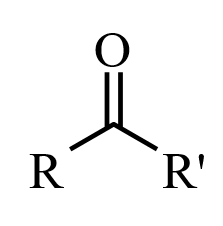
The ketone carbon is sp2 hybridized, and it adopts a trigonal planar geometry around the ketonic carbon. As such, the [latex]\text{C–C–O}[/latex] and [latex]\text{C–C–C}[/latex] bond angles are approximately 120 degrees. Due to the carbonyl group, ketones are polar and are able to interact with other compounds through hydrogen bonding; this hydrogen bond capability makes ketones more soluble in water than related methylene compounds. Ketones are not usually hydrogen bond donors, and they tend not to exhibit intermolecular attractions with other ketones. As a result, ketones are often more volatile than alcohols and carboxylic acids of comparable molecular weights. Ketones have alpha -hydrogens which participate in keto-enol tautomerism. In the presence of a strong base, enolate formation and subsequent deprotonation of the enolate will occur.
Aldehydes
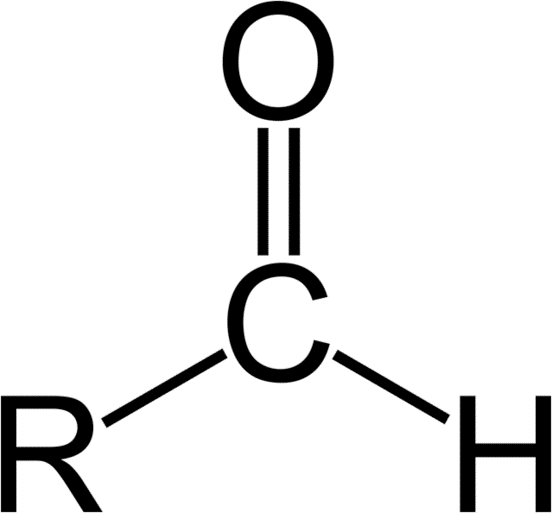
An aldehyde is an organic compound that contains a carbonyl group with the central carbon bonded to a hydrogen and R group (R-CHO). Aldehydes differ from ketones in that the carbonyl is placed at the end of the carbon skeleton rather than between two carbon atoms of the backbone. Like ketones, aldehydes are sp2 hybridized and can exist in the keto or enol tautomer. Aldehydes are named by dropping the suffix of the parent molecule, and adding the suffix “-al.” For instance, a three-carbon chain with an aldehyde group on a terminal carbon would be propanal. If there are higher order functional groups on the compound, the prefix “oxo-” can be used to indicate which carbon atom is part of the aldehyde group. If the location of the aldehyde must be specified, a number can be used in between the parent chain and suffix, or at the beginning of the compound name.
Similarities of Aldehydes and Ketones
Both aldehydes and ketones exist in an equilibrium with their enol forms; the enol form is defined as an alkene with a hydroxyl group affixed to one of the carbon atoms composing the double bond. The keto form predominates at equilibrium for most ketones. However, the enol form is important for some reactions because the deprotonated enolate form is a strong nucleophile. The equilibrium is strongly thermodynamically driven, and at room temperature the keto form is favored. The interconversion can be catalyzed by the presence of either an acid or a base.
Ketone and Aldehyde Spectroscopy
Both ketones and aldehydes can be identified by spectroscopic methods. They display strong [latex]\text{CO}[/latex] absorption bands near 1700 cm-1. In NMR spectroscopy, the carbonyl hydrogen shows a strong absorption peak, and any coupling to protons on the alpha carbon will also show strong signals.
Ketones and aldehydes can both be readily reduced to alcohols, usually in the presence of a strong reducing agent such as sodium borohydride. In the presence of strong oxidizing agents, they can be oxidized to carboxylic acids. As electrophiles, they are subject to attack by nucleophiles, meaning they participate in many nucleophilic addition reactions.
Carboxylic Acids
Carboxylic acids are organic acids that contain a carbon atom that participates in both a hydroxyl and a carbonyl functional group.
LEARNING OBJECTIVES
Recognize the general properties of carboxylic acids
KEY TAKEAWAYS
Key Points
- Carboxylic acids are used as precursors to form other compounds such as esters, aldehydes, and ketones.
- Carboxylic acids can exhibit hydrogen bonding with themselves, especially in non- polar solvents; this leads to increased stabilization of the compounds and elevates their boiling points.
- Since they contain both hydroxyl and carbonyl functional groups, carboxylic acids participate in hydrogen bonding as both hydrogen acceptors and hydrogen donors.
Key Terms
- nitrile: Any of a class of organic compounds containing a cyano functional group ([latex]\text{-C}\equiv{N}[/latex]).
- olefin: Any of a class of unsaturated, open-chain hydrocarbons such as ethylene; an alkene with only one carbon-carbon double bond.
- ester: A compound most often formed by the condensation of an alcohol and an acid, with elimination of water. It contains the functional group C=O joined via carbon to another oxygen atom.
A carboxyl group ([latex]\text{COOH}[/latex]) is a functional group consisting of a carbonyl group ([latex]\text{C=O}[/latex]) with a hydroxyl group ([latex]\text{O-H}[/latex]) attached to the same carbon atom. Carboxyl groups have the formula [latex]\text{-C(=O)OH}[/latex], usually written as [latex]\text{-COOH}[/latex] or [latex]\text{CO}_2\text{H}[/latex]. Carboxylic acids are a class of molecules which are characterized by the presence of one carboxyl group. As proton donors, carboxylic acids are characterized as Brønsted-Lowry acids. Acids with two or more carboxylic groups are called dicarboxylic, tricarboxylic, etc. Salts and esters of carboxylic acids are called carboxylates. Carboxylate ions are resonance-stabilized. This increased stability leads to increased acidity compared to that of alcohols. Generally, in IUPAC nomenclature, carboxylic acids have an “-oic acid ” suffix, although “-ic acid” is the suffix most commonly used.
Physical Properties of Carboxylic Acids
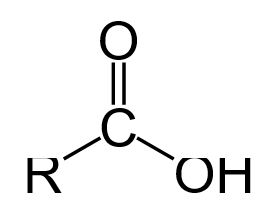
Carboxylic acids act as both hydrogen bond acceptors, due to the carbonyl group, and hydrogen bond donors, due to the hydroxyl group. As a result, they often participate in hydrogen bonding. Carboxylic acids usually exist as dimeric pairs in nonpolar media because of their tendency to “self-associate.” This tendency to hydrogen bond gives them increased stability as well as higher boiling points relative to the acid in aqueous solution. Carboxylic acids are polar molecules; they tend to be soluble in water, but as the alkyl chain gets longer, their solubility decreases due to the increasing hydrophobic nature of the carbon chain. Carboxylic acids are characterized as weak acids, meaning that they do not fully dissociate to produce H+ cations in a neutral aqueous solution.
Spectroscopy of Carboxylic Acids
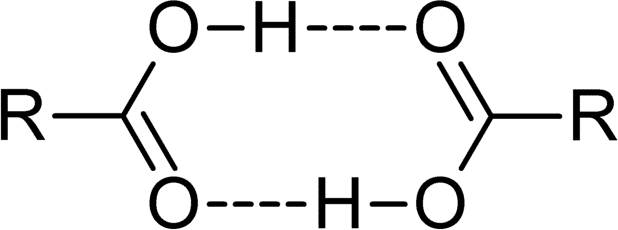
Carboxylic acids can be characterized by IR spectroscopy; they exhibit a sharp band associated with vibration of the [latex]\text{C-O}[/latex] bond between 1680 and 1725 cm-1. Additionally, a broad peak appears in the 2500 to 3000 cm-1 region. By [latex]^1\text{H NMR}[/latex] spectroscopy, the hydroxyl hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.
Applications and Reactivity of Carboxylic Acids
Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, and food additives. As such, they are often produced industrially on a large scale. Carboxylic acids are generally produced from oxidation of aldehydes and hydrocarbons, and base catalyzed dehydrogenation of alcohols. They can be produced in the laboratory for small scale reactions via the oxidation of primary alcohols or aldehydes, oxidative cleavage of olefins, and through the hydrolysis of nitriles, esters, or amides.
Carboxylic acids are widely used as precursors to produce other compounds. Upon exposure to a base, the carboxylic acid is deprotonated and forms a carboxylate salt. They also react with alcohols to produce esters and can undergo reduction reactions by hydrogenation or the use of reducing agents. There are also various specialized reactions that carboxylic acids participate in that lead to the formation of amines, aldehydes, and ketones.
Esters
Esters are functional groups produced from the condensation of an alcohol with a carboxylic acid, and are named based on these components.
LEARNING OBJECTIVES
Identify the general properties of the ester functional group
KEY TAKEAWAYS
Key Points
- Esters are a functional group commonly encountered in organic chemistry. They are characterized by a carbon bound to three other atoms: a single bond to a carbon, a double bond to an oxygen, and a single bond to an oxygen. The singly bound oxygen is bound to another carbon.
- Ester names are derived from the parent alcohol and the parent acid. While simple esters are often called by their common names, all esters can be named using the systematic IUPAC name, based on the name for the acid followed by the suffix “-oate.”
- Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic, but is attacked by strong nucleophiles. The [latex]\text{C-H}[/latex] bonds adjacent to the carbonyl are weakly acidic, but undergo deprotonation with strong bases.
Key Terms
- carboxylic acid: Any of a class of organic compounds containing a carboxyl functional group—a carbon with one double bond to an oxygen and a single bond to another oxygen, which is in turn bonded to a hydrogen.
- alcohol: Class of organic compounds containing a hydroxyl functional group.
- nucleophile: A compound or functional group that is attractive to centers of positive charge and donates electrons; donates an electron pair to an electrophile to form a bond.
Esters are an important functional group in organic chemistry, and they are generally written [latex]\text{RCOOR’}[/latex] or [latex]\text{RCO}_2\text{R’}[/latex].
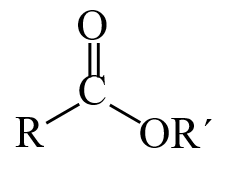
As usual, [latex]\text{R}[/latex] and [latex]\text{R’}[/latex] are both alkyl groups or groups initiating with carbon. Esters are derivative of carboxylic acids where the hydroxyl ([latex]\text{OH}[/latex]) group has been replaced by an alkoxy ([latex]\text{O-R}[/latex]) group. They are commonly synthesized from the condensation of a carboxylic acid with an alcohol:
[latex]\text{RCO}_2\text{H} + \text{R'OH} \rightarrow \text{RCO}_2\text{R'} + \text{H}_2\text{O}[/latex]
Esters are ubiquitous. Most naturally occurring fats and oils are the fatty acid esters of glycerol. Esters are typically fragrant, and those with low enough molecular weights to be volatile are commonly used as perfumes and are found in essential oils and pheromones. Polymerized esters, or polyesters, are important plastics, with monomers linked by esteric units like this:
[latex]\text{CO}_2\text{RCO}_2\text{RCO}_2\text{R... etc}[/latex]
Nomenclature
The word “ester” was coined in 1848 by German chemist Leopold Gmelin, probably as a contraction of the German Essigäther, meaning acetic ether.
Ester names are derived from the parent alcohol and acid. For example, the ester formed by ethanol and ethanoic acid is known as ethyl ethanoate; “ethanol” is reduced to “ethyl,” while “ethanoic acid” is reduced to “ethanoate.” Other examples of ester names include methyl propanoate, from methanol and propanoic acid, and butyl octanoate, from butane and octanoic acid.
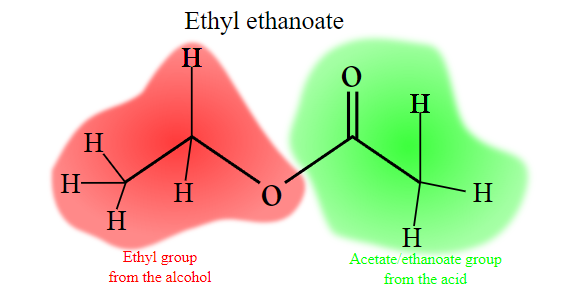
In the case of esters formed from common carboxylic acids, more colloquial terms are sometimes used. For example, ethanoic acid is more commonly known as acetic acid, and thus its esters contain “acetate” instead of “ethanoate” in their names. Other such substitutions include “formate” instead of “methanoate,” “propionate” instead of “propanoate,” and “butyrate” instead of “butanoate.”
The chemical formulas of organic esters are typically written in the format of [latex]\text{RCO}_2\text{R’}[/latex], where [latex]\text{R}[/latex] and [latex]\text{R’}[/latex] are the hydrocarbon parts of the carboxylic acid and alcohol, respectively. For example, butyl acetate, systematically known as ethanoic acid, is derived from butanol and acetic acid and would be written [latex]\text{CH}_3\text{CO}_2\text{C}_4\text{H}_9[/latex]. Alternative presentations are common, including [latex]\text{BuOAc}[/latex] and [latex]\text{CH}_3\text{COOC}_4\text{H}_9[/latex]. Cyclic esters are known as lactones.
Structure and Bonding
Esters contain a carbonyl center, which gives rise to 120 degree [latex]\text{C-C-O}[/latex] and [latex]\text{O-C-O}[/latex] bond angles due to sp2hybridization. Unlike amides, esters are structurally flexible functional groups because rotation about the [latex]\text{C-O-C}[/latex] bonds has a lower energy barrier. Their flexibility and low polarity affects their physical properties on a macroscopic scale; they tend to be less rigid, leading to a lower melting point, and more volatile, leading to a lower boiling point, than the corresponding amides. The [latex]\text{pK}_a[/latex] of the alpha-hydrogens, or the hydrogens attached to the carbon adjacent to the carbonyl, on esters is around 25, making them essentially non-acidic except in the presence of very strong bases.
Physical Properties and Characterization
Esters are more polar than ethers, but less so than alcohols. They participate in hydrogen bonds as hydrogen bond acceptors, but cannot act as hydrogen bond donors, unlike their parent alcohols and carboxylic acids. This ability to participate in hydrogen bonding confers some water-solubility, depending on the length of the alkyl chains attached. Since they have no hydrogens bonded to oxygens, as alcohols and carboxylic acids do, esters do not self-associate. Consequently, esters are more volatile than carboxylic acids of similar molecular weight.
Characterization and Analysis
Esters are usually identified by gas chromatography, taking advantage of their volatility. IR (infrared) spectra for esters feature an intense, sharp band in the range 1730–1750 cm−1 assigned to [latex]\text{vC=O}[/latex], or vibration of the C=O bond. This peak changes depending on the functional groups attached to the carbonyl. For example, a benzene ring or double bond in conjugation with the carbonyl will bring the wavenumber down to around 30 cm−1.
Reactivity
Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic, but is attacked by strong nucleophiles such as amines, alkoxides, hydride sources, and organolithium compounds. The carbonyl’s electrophilicity can increase if it is protonated; in acidic media, an ester can be hydrolyzed by water to form a carboxylic acid and an alcohol.
The C-H bonds adjacent to the carbonyl are weakly acidic, but undergo deprotonation with strong bases. This process is the one that usually initiates condensation reactions. The carbonyl oxygen is weakly basic (less so than in amides), but can form adducts with Lewis acids.
Amines
Amines are compounds characterized by the presence of a nitrogen atom, a lone pair of electrons, and three substituents.
LEARNING OBJECTIVES
Identify the general properties of amines
KEY TAKEAWAYS
Key Points
- Due to the lone pair of electrons, amines are basic compounds. The basicity of the compound can be influenced by neighboring atoms, steric bulk, and the solubility of the corresponding cation to be formed.
- Amine compounds can hydrogen bond, which affords them solubility in water and elevated boiling points.
- The general structure of an amine is a nitrogen atom with a lone pair of electrons and three substituents. However, the nitrogen may bind to four substituents, leaving a positive charge on the nitrogen atom. These charged species can serve as intermediates for important reactions.
Key Terms
- aliphatic: Of a class of organic compounds in which the carbon atoms are arranged in an open chain.
- chiral: Literally, “handedness;” refers to a compound in which central atoms are bonded to multiple different substituents, such that the mirror image of the compound is not identical to the original.
- amine: Organic compounds or the functional group that contains a basic nitrogen atom with a lone pair.
- inversion: For a secondary or tertiary amine bonded to three different substitutents, the flipping of the three bonds about the central [latex]\text{N}[/latex] atom, resulting in the opposite stereoisomer.
The amine functional group contains a basic nitrogen atom with a lone pair of electrons. As such, the group is derivative of ammonia, in which one or more hydrogen atoms have been replaced by a carbon-containing substituent. Compounds with the nitrogen group attached to a carbonyl within the structure are referred to as amides, and they have the structure [latex]\text{R-CO-NR’R”}[/latex]. Amine groups bonded to an aromatic (conjugated cyclic) structure are known as aromatic amines. The aromatic structure effectively decreases the alkalinity of the amine, while the presence of the amine group significantly decreases the reactivity of the ring due to an electron donating effect. The prefix “amino-” or the suffix “-amine” is used when naming an amine compound. An organic compound with multiple amino groups is called a diamine, triamine, tetramine, etc.
Amine Structure
Amines are generally organized into categories based on their bonding environments. Amines that have one of their three hydrogen atoms replaced by an alkyl or aromatic substituent are referred to as primary amines. Secondary amines are those that have two substituents and one hydrogen bonded to a nitrogen. Tertiary amines are amines whose hydrogens have been completely replaced by organic substituents. Finally, cyclic amines are those in which the nitrogen has been incorporated into a ring structure, effectively making it either a secondary or tertiary amine. The general structure of an amine contains a nitrogen atom, a lone pair of electrons, and three substituents. However, it is possible to have four organic substituents on the nitrogen, making it an ammonium cation with a charged nitrogen center.
Physical Properties of Amines
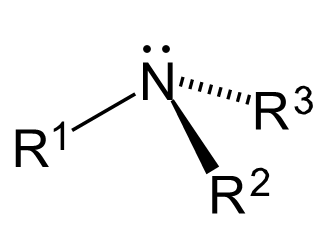
Amines are able to hydrogen bond. As a result, the boiling points of these compounds are higher than those of the corresponding phosphines, but lower than those of the corresponding alcohols, which hydrogen bond to a stronger extent. Amines also display some solubility in water. However, the solubility decreases with an increase in carbon atoms, due to the increased hydrophobicity of the compound as the chain length increases. Aliphatic amines, which are amines connected to an alkyl chain, display solubility in organic polar solvents. Aromatic amines, which are amines that participate in a conjugated ring, donate their lone pair of electrons into the benzene ring, and thus their ability to engage in hydrogen bonding decreases. This results in a decrease in their solubility in water and high boiling points.
Acidity and Alkalinity of Amines
Amines of the type [latex]\text{NHRR’}[/latex] and [latex]\text{NR’R”R”’}[/latex] are chiral molecules and can undergo inversion. Since the barrier for inversion is quite low (~7 kcal/mol), these compounds cannot be resolved optically. Amines are bases, and their basicity depends on the electronic properties of the substituents (alkyl groups enhance the basicity; aryl groups diminish it), steric hindrance, and the degree of solvation of the protonated amine. In general, the effect of alkyl groups raises the energy of the lone pair of electrons, thus elevating the basicity. Thus, the basicity of an amine can be expected to increase with the number of alkyl groups on the amine. Additionally, the effect of the aromatic ring delocalizes the lone pair of electrons on nitrogen into the ring, resulting in decreased basicity. The solvation of protonated amines changes upon their conversion to ammonium compounds. Typically, salts of ammonium compounds exhibit the following order of solubility in water: primary ammonium ([latex]\text{RNH}_3^+[/latex]) > secondary ammonium ([latex]\text{R}_2\text{NH}_2^+[/latex]) > tertiary ammonium ([latex]\text{R}_3\text{NH}^+[/latex]). Quaternary ammonium salts usually exhibit the lowest solubility of the series.
Amine Preparation and Reactivity
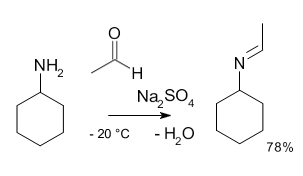
Industrially, amines are prepared from ammonia by alkylation with alcohols. They can also be prepared via reduction of nitriles to amines using hydrogen in the presence of a nickel catalyst. Amines are quite reactive due to their basicity as well as their nucleophilicity. Most primary amines are good ligands and react with metal ions to yield coordination complexes. One of the most important reactions for amines is their formation of imines, or organic compounds where nitrogen participates in a double bond, upon reacting with ketones or aldehydes.
Applications of Amines
Amines are ubiquitous in biology. Many important molecules are amine-based, such as neurotransmitters and amino acids. Their applications in the world include being starting material for dyes and models for drug design. They are also used for gas treatment, such as removing [latex]\text{CO}_2[/latex] from combustion gases.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- functional group. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/functional_group. License: CC BY-SA: Attribution-ShareAlike
- Functional groups. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Functional_groups. License: CC BY-SA: Attribution-ShareAlike
- functionalization. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/functionalization. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- carboxylic acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/carboxylic_acid. License: CC BY-SA: Attribution-ShareAlike
- Alcohol. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alcohol. License: CC BY-SA: Attribution-ShareAlike
- alkane. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alkane. License: CC BY-SA: Attribution-ShareAlike
- aldehyde. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/aldehyde. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- Ethers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ethers. License: CC BY-SA: Attribution-ShareAlike
- amide. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/amide. License: CC BY-SA: Attribution-ShareAlike
- ester. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ester. License: CC BY-SA: Attribution-ShareAlike
- ether. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ether. License: CC BY-SA: Attribution-ShareAlike
- alkene. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alkene. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- tetrahydrofuran. Provided by: wikipedia. Located at: http://en.wikipedia.org/wiki/Tetrahydrofuran. License: CC BY-SA: Attribution-ShareAlike
- Ether-(general). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ether-(general).png. License: Public Domain: No Known Copyright
- Ketone. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ketone. License: CC BY-SA: Attribution-ShareAlike
- Keto-enol tautomerism. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Keto-enol_tautomerism. License: CC BY-SA: Attribution-ShareAlike
- Carbonyl. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Carbonyl. License: CC BY-SA: Attribution-ShareAlike
- Aldehyde. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Aldehyde. License: CC BY-SA: Attribution-ShareAlike
- tautomerism. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/tautomerism. License: CC BY-SA: Attribution-ShareAlike
- Boundless. Provided by: Boundless Learning. Located at: http://www.boundless.com//chemistry/definition/sp2. License: CC BY-SA: Attribution-ShareAlike
- ketone. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ketone. License: CC BY-SA: Attribution-ShareAlike
- oxidize. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/oxidize. License: CC BY-SA: Attribution-ShareAlike
- organic chemistry. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/organic_chemistry. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- tetrahydrofuran. Provided by: wikipedia. Located at: http://en.wikipedia.org/wiki/Tetrahydrofuran. License: CC BY-SA: Attribution-ShareAlike
- Ether-(general). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ether-(general).png. License: Public Domain: No Known Copyright
- File:Ketone-group-2D-skeletal.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ketone-group-2D-skeletal.svg&page=1. License: Public Domain: No Known Copyright
- Enol. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Enol.png. License: Public Domain: No Known Copyright
- File:Keto-enol.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Keto-enol.svg&page=1. License: Public Domain: No Known Copyright
- Aldehyde2. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Aldehyde2.png. License: Public Domain: No Known Copyright
- Carboxylic acid. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Carboxylic_acid. License: CC BY-SA: Attribution-ShareAlike
- nitrile. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nitrile. License: CC BY-SA: Attribution-ShareAlike
- olefin. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/olefin. License: CC BY-SA: Attribution-ShareAlike
- ester. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/ester. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- tetrahydrofuran. Provided by: wikipedia. Located at: http://en.wikipedia.org/wiki/Tetrahydrofuran. License: CC BY-SA: Attribution-ShareAlike
- Ether-(general). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ether-(general).png. License: Public Domain: No Known Copyright
- File:Ketone-group-2D-skeletal.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ketone-group-2D-skeletal.svg&page=1. License: Public Domain: No Known Copyright
- Enol. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Enol.png. License: Public Domain: No Known Copyright
- File:Keto-enol.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Keto-enol.svg&page=1. License: Public Domain: No Known Copyright
- Aldehyde2. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Aldehyde2.png. License: Public Domain: No Known Copyright
- Carboxylic%20acid%20dimers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Carboxylic_acid_dimers.png. License: Public Domain: No Known Copyright
- File:Carboxylic-acid.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Carboxylic-acid.svg&page=1. License: Public Domain: No Known Copyright
- carboxylic acid. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/carboxylic_acid. License: CC BY-SA: Attribution-ShareAlike
- Ester. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Ester. License: CC BY-SA: Attribution-ShareAlike
- alkyl. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alkyl. License: CC BY-SA: Attribution-ShareAlike
- nucleophile. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nucleophile. License: CC BY-SA: Attribution-ShareAlike
- alcohol. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alcohol. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- tetrahydrofuran. Provided by: wikipedia. Located at: http://en.wikipedia.org/wiki/Tetrahydrofuran. License: CC BY-SA: Attribution-ShareAlike
- Ether-(general). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ether-(general).png. License: Public Domain: No Known Copyright
- File:Ketone-group-2D-skeletal.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ketone-group-2D-skeletal.svg&page=1. License: Public Domain: No Known Copyright
- Enol. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Enol.png. License: Public Domain: No Known Copyright
- File:Keto-enol.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Keto-enol.svg&page=1. License: Public Domain: No Known Copyright
- Aldehyde2. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Aldehyde2.png. License: Public Domain: No Known Copyright
- Carboxylic%20acid%20dimers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Carboxylic_acid_dimers.png. License: Public Domain: No Known Copyright
- File:Carboxylic-acid.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Carboxylic-acid.svg&page=1. License: Public Domain: No Known Copyright
- Ethylethanoate-parts. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ethylethanoate-parts.svg. License: Public Domain: No Known Copyright
- File:Ester-general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ester-general.svg&page=1. License: Public Domain: No Known Copyright
- Amines. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Amines. License: CC BY-SA: Attribution-ShareAlike
- chiral. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/chiral. License: CC BY-SA: Attribution-ShareAlike
- aliphatic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/aliphatic. License: CC BY-SA: Attribution-ShareAlike
- amine. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/amine. License: CC BY-SA: Attribution-ShareAlike
- aromatic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/aromatic. License: CC BY-SA: Attribution-ShareAlike
- Alcohol examples. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Alcohol_examples.gif. License: Public Domain: No Known Copyright
- Organic chemistry functional groups lesson. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Alcohol general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Alcohol_general.svg&page=1. License: Public Domain: No Known Copyright
- tetrahydrofuran. Provided by: wikipedia. Located at: http://en.wikipedia.org/wiki/Tetrahydrofuran. License: CC BY-SA: Attribution-ShareAlike
- Ether-(general). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ether-(general).png. License: Public Domain: No Known Copyright
- File:Ketone-group-2D-skeletal.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ketone-group-2D-skeletal.svg&page=1. License: Public Domain: No Known Copyright
- Enol. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Enol.png. License: Public Domain: No Known Copyright
- File:Keto-enol.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Keto-enol.svg&page=1. License: Public Domain: No Known Copyright
- Aldehyde2. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Aldehyde2.png. License: Public Domain: No Known Copyright
- Carboxylic%20acid%20dimers. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Carboxylic_acid_dimers.png. License: Public Domain: No Known Copyright
- File:Carboxylic-acid.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Carboxylic-acid.svg&page=1. License: Public Domain: No Known Copyright
- Ethylethanoate-parts. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Ethylethanoate-parts.svg. License: Public Domain: No Known Copyright
- File:Ester-general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Ester-general.svg&page=1. License: Public Domain: No Known Copyright
- N-Ethylidenecyclohexylamine. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:N-Ethylidenecyclohexylamine.svg. License: CC BY-SA: Attribution-ShareAlike
- File:Amine-2D-general.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Amine-2D-general.svg&page=1. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter “Functional Group Names, Properties, Reactions” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
part of an organic molecule that imparts a specific chemical reactivity to the molecule
Addition of specific functional groups to afford the compound new, desirable properties.
molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
Any of a large class of reactive organic compounds ([latex]\text{RCHO}[/latex]) having a carbonyl functional group attached to one hydrocarbon radical and a hydrogen atom.
Any of a class of organic compounds containing a carboxyl functional group—a carbon with a double bond to an oxygen and a single bond to another oxygen, which is in turn bonded to a hydrogen.
In organic chemistry, the species that leaves the parent molecule following a substitution reaction.
molecule consisting of carbon and hydrogen containing at least one carbon-carbon double bond
a compound usually formed by condensing an alcohol and an acid and eliminating of water. it contains the functional group carbon-oxygen double bond joined via carbon to another oxygen atom
Compound containing an oxygen atom bonded to two hydrocarbon groups.
Any derivative of an oxoacid in which the hydroxyl group has been replaced with an amino or substituted amino group; especially such derivatives of a carboxylic acid, the carboxamides.
A form of isomerism in which a dynamic equilibrium between multiple isomers exists, such as that between an enol and a ketone.
To increase the valence (the positive charge) of an element by removing electrons.
Hybrid orbital that forms when one pi bond is required for the double bond, and only three σ bonds are formed per carbon atom. The 2s orbital is mixed with only two of the three 2p orbitals.
A compound containing an oxygen atom joined to a carbon atom by a double bond.
Any of a class of organic compounds containing a cyano functional group ([latex]\text{-C} \equiv \text{N}[/latex]).
Any of a class of unsaturated, open-chain hydrocarbons such as ethylene; an alkene with only one carbon-carbon double bond.
Class of organic compounds containing a hydroxyl functional group.
A compound or functional group that is attractive to centers of positive charge and donates electrons; donates an electron pair to an electrophile to form a bond.
Of a class of organic compounds in which the carbon atoms are arranged in an open chain.
Literally, “handedness;” refers to a compound in which central atoms are bonded to multiple different substituents, such that the mirror image of the compound is not identical to the original.
Organic compounds or the functional group that contains a basic nitrogen atom with a lone pair.
For a secondary or tertiary amine bonded to three different substitutents, the flipping of the three bonds about the central N atom, resulting in the opposite stereoisomer.

