10 Energy
LumenLearning
Types of Energy
The various types of energy include kinetic, potential, and chemical energy.
LEARNING OBJECTIVES
Differentiate between types of energy.
KEY TAKEAWAYS
Key Points
- All organisms use different forms of energy to power the biological processes that allow them to grow and survive.
- Kinetic energy is the energy associated with objects in motion.
- Potential energy is the type of energy associated with an object’s potential to do work.
- Chemical energy is the type of energy released from the breakdown of chemical bonds and can be harnessed for metabolic processes.
Key Terms
- chemical energy: The net potential energy liberated or absorbed during the course of a chemical reaction.
- potential energy: Energy possessed by an object because of its position (in a gravitational or electric field) or its condition (as a stretched or compressed spring, as a chemical reactant, or by having rest mass).
- kinetic energy: The energy possessed by an object because of its motion, equal to one half the mass of the body times the square of its velocity.
Energy is a property of objects which can be transferred to other objects or converted into different forms but cannot be created or destroyed. Organisms use energy to survive, grow, respond to stimuli, reproduce, and for every type of biological process. The potential energy stored in molecules can be converted to chemical energy, which can ultimately be converted to kinetic energy, enabling an organism to move. Eventually, most of energy used by organisms is transformed into heat and dissipated.
Kinetic Energy
Energy associated with objects in motion is called kinetic energy. For example, when an airplane is in flight, the airplane is moving through air very quickly—doing work to enact change on its surroundings. The jet engines are converting potential energy in fuel to the kinetic energy of movement. A wrecking ball can perform a large amount of damage, even when moving slowly. However, a still wrecking ball cannot perform any work and therefore has no kinetic energy. A speeding bullet, a walking person, the rapid movement of molecules in the air that produces heat, and electromagnetic radiation, such as sunlight, all have kinetic energy.
Potential Energy
What if that same motionless wrecking ball is lifted two stories above a car with a crane? If the suspended wrecking ball is not moving, is there energy associated with it? Yes, the wrecking ball has energy because the wrecking ball has the potential to do work. This form of energy is called potential energy because it is possible for that object to do work in a given state.
Objects transfer their energy between potential and kinetic states. As the wrecking ball hangs motionlessly, it has kinetic and 100% potential energy. Once the ball is released, its kinetic energy increases as the ball picks up speed. At the same time, the ball loses potential energy as it nears the ground. Other examples of potential energy include the energy of water held behind a dam or a person about to skydive out of an airplane.

Potential energy vs. kinetic energy: Water behind a dam has potential energy. Moving water, such as in a waterfall or a rapidly flowing river, has kinetic energy.
Chemical Energy
Potential energy is not only associated with the location of matter but also with the structure of matter. A spring on the ground has potential energy if it is compressed, as does a rubber band that is pulled taut. The same principle applies to molecules. On a chemical level, the bonds that hold the atoms of molecules together have potential energy. This type of potential energy is called chemical energy, and like all potential energy, it can be used to do work.
For example, chemical energy is contained in the gasoline molecules that are used to power cars. When gas ignites in the engine, the bonds within its molecules are broken and the energy released is used to drive the pistons. The potential energy stored within chemical bonds can be harnessed to perform work for biological processes. Different metabolic processes break down organic molecules to release the energy for an organism to grow and survive.
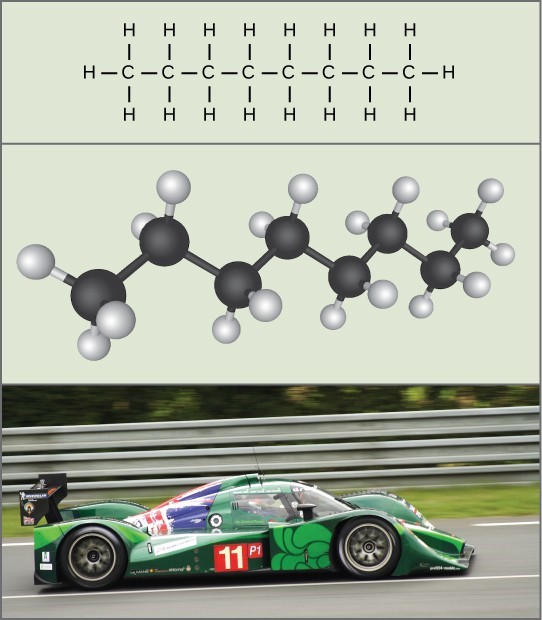
Chemical energy: The molecules in gasoline (octane, the chemical formula shown) contain chemical energy. This energy is transformed into kinetic energy that allows a car to drive on a racetrack.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44425/latest/?collection=col11448/latest. License: CC BY: Attribution
- kinetic energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/kinetic%20energy. License: CC BY-SA: Attribution-ShareAlike
- potential energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/potential%20energy. License: CC BY-SA: Attribution-ShareAlike
- Energy. Provided by: Wikipedia. Located at: https://en.wikipedia.org/wiki/Energy. License: CC BY-SA: Attribution-ShareAlike
- chemical energy. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/chemical_energy. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44425/latest/Figure_06_03_01ab.jpg. License: CC BY: Attribution
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44425/latest/Figure_06_03_02ab.jpg. License: CC BY: Attribution
- Chemical reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_reaction. License: CC BY-SA: Attribution-ShareAlike
- enthalpy. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/enthalpy. License: CC BY-SA: Attribution-ShareAlike
- exothermic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/exothermic. License: CC BY-SA: Attribution-ShareAlike
- endothermic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/endothermic. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44425/latest/Figure_06_03_01ab.jpg. License: CC BY: Attribution
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44425/latest/Figure_06_03_02ab.jpg. License: CC BY: Attribution
- Chemical reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Chemical_reaction. License:
- Electrolysis of Water. Provided by: WikiPedia. Located at: http://en.wikipedia.org/wiki/Electrolysis_of_water%23mediaviewer/File:Electrolysis_of_Water.png. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter “Energy” in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
The net potential energy liberated or absorbed during the course of a chemical reaction.
energy of a particle or system of particles derived from relative position, composition, or condition
energy of a moving body, in joules, equal to 12mv212mv2 (where m = mass and v = velocity)

