72 Electrolysis
OpenStax
LEARNING OBJECTIVES
By the end of this section you will be able to:
- Describe the process of electrolysis
- Compare the operation of electrolytic cells with that of galvanic cells
Electrochemical cells in which spontaneous redox reactions take place (galvanic cells) have been the topic of discussion so far in this chapter. In these cells, electrical work is done by a redox system on its surroundings as electrons produced by the redox reaction are transferred through an external circuit. This final section of the chapter will address an alternative scenario in which an external circuit does work on a redox system by imposing a voltage sufficient to drive an otherwise nonspontaneous reaction, a process known as electrolysis. A familiar example of electrolysis is recharging a battery, which involves use of an external power source to drive the spontaneous (discharge) cell reaction in the reverse direction, restoring to some extent the composition of the half-cells and the voltage of the battery. Perhaps less familiar is the use of electrolysis in the refinement of metallic ores, the manufacture of commodity chemicals, and the electroplating of metallic coatings on various products (e.g., jewelry, utensils, auto parts). To illustrate the essential concepts of electrolysis, a few specific processes will be considered.
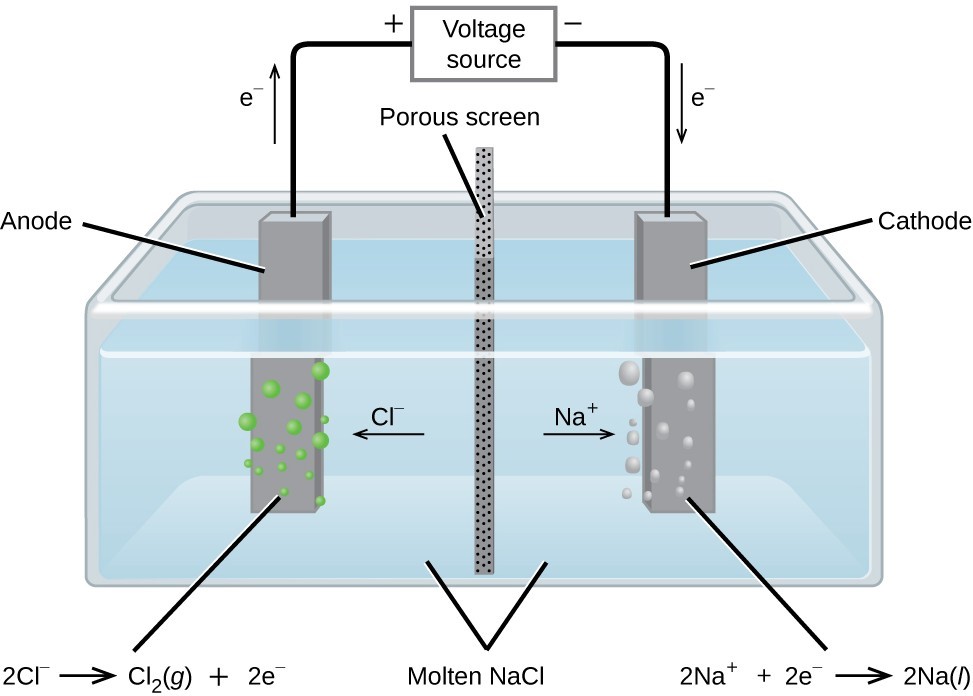
The Electrolysis of Molten Sodium Chloride
Metallic sodium, [latex]\text{Na}[/latex], and chlorine gas, [latex]\text{Cl}_2[/latex], are used in numerous applications, and their industrial production relies on the large-scale electrolysis of molten sodium chloride, [latex]\text{NaCl} (l)[/latex]. The industrial process typically uses a Downs cell similar to the simplified illustration shown in the figure below. The reactions associated with this process are:
| [latex]\text{anode:}[/latex] | [latex]\text{2Cl} (l) \rightarrow \text{Cl}_2 (g) + \text{2e}[/latex] |
| [latex]\text{cathode:}[/latex] | [latex]\text{Na}^+ (l) + \text{e}^-\rightarrow \text{Na} (l)[/latex] |
| |
|
| [latex]\text{cell:}[/latex] | [latex]\text{2Na}^+ (l) + \text{2Cl}^- (l) \rightarrow \text{2Na} (l) + \text{Cl}_2 (g)[/latex] |
The cell potential for the above process is negative, indicating the reaction as written (decomposition of liquid [latex]\text{NaCl}[/latex]) is not spontaneous. To force this reaction, a positive potential of magnitude greater than the negative cell potential must be applied to the cell.
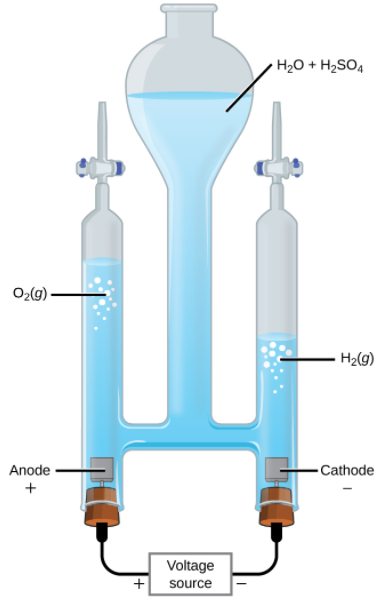
The Electrolysis of Water
Water may be electrolytically decomposed in a cell similar to the one illustrated in the figure above. To improve electrical conductivity without introducing a different redox species, the hydrogen ion concentration of the water is typically increased by addition of a strong acid. The redox processes associated with this cell are
| [latex]\text{anode:}[/latex] | [latex]\text{2H}_2\text{O} (l) \rightarrow \text{O}_2 (g) + \text{4H}^+ (aq) + \text{4e}^- [/latex] | [latex]\text{E}_\text{anode} ^\circ \text{= +1.229 V}[/latex] |
| [latex]\text{cathode:}[/latex] | [latex]\text{2H}^+ (aq) + \text{2e}^- \rightarrow \text{H}_2 (g)[/latex] | [latex]\text{E}_\text{cathode} ^\circ \text{= 0 V}[/latex] |
| |
||
| [latex]\text{cell:}[/latex] | [latex]\text{2H}_2\text{O} (l) \rightarrow \text{2H}_2 (g) + \text{O}_2 (g)[/latex] | [latex]\text{E}_\text{cell} ^\circ \text{= -1.229 V}[/latex] |
Again, the cell potential as written is negative, indicating a nonspontaneous cell reaction that must be driven by imposing a cell voltage greater than +1.229 V. Keep in mind that standard electrode potentials are used to inform thermodynamic predictions here, though the cell is not operating under standard state conditions. Therefore, at best, calculated cell potentials should be considered ballpark estimates.
The Electrolysis of Aqueous Sodium Chloride
When aqueous solutions of ionic compounds are electrolyzed, the anode and cathode half-reactions may involve the electrolysis of either water species [latex]\text{(H}_2\text{O, H}^+\text{, OH}^-)[/latex] or solute species (the cations and anions of the compound). As an example, the electrolysis of aqueous sodium chloride could involve either of these two anode reactions:
| [latex]\text{(i) 2Cl}^- (aq) \rightarrow \text{Cl}_2 (g) + \text{2e}^- [/latex] | [latex]\text{E}_\text{anode} ^\circ \text{= +1.35827 V}[/latex] |
| [latex]\text{(ii) 2H}_2\text{O} (l) \rightarrow \text{O}_2 (g) + \text{4H}^+ (aq) + \text{4e}^- [/latex] | [latex]\text{E}_\text{anode} ^\circ \text{= +1.229 V}[/latex] |
The standard electrode (reduction) potentials of these two half-reactions indicate water may be oxidized at a less negative/more positive potential (–1.229 V) than chloride ion (–1.358 V). Thermodynamics thus predicts that water would be more readily oxidized, though in practice it is observed that both water and chloride ion are oxidized under typical conditions, producing a mixture of oxygen and chlorine gas.
Turning attention to the cathode, the possibilities for reduction are:
| [latex]\text{(iii) 2H}^+ (aq) + \text{2e}^- \rightarrow \text{H}_2 (g)[/latex] | [latex]\text{E}_\text{cathode} ^\circ \text{= 0 V}[/latex] |
| [latex]\text{(iv) 2H}_2\text{O} (l) + \text{2e}^- \rightarrow \text{H}_2 (g) + \text{2OH}^- (aq)[/latex] | [latex]\text{E}_\text{cathode} ^\circ \text{= -0.8277 V}[/latex] |
| [latex]\text{(v) Na}^+ (aq) + \text{e}^- \rightarrow \text{Na} (s) [/latex] | [latex]\text{E}_\text{cathode} ^\circ \text{= -2.71 V}[/latex] |
Comparison of these standard half-reaction potentials suggests the reduction of hydrogen ion is thermodynamically favored. However, in a neutral aqueous sodium chloride solution, the concentration of hydrogen ion is far below the standard state value of 1 M (approximately 10-7 M), and so the observed cathode reaction is actually reduction of water. The net cell reaction in this case is then
| [latex]\text{cell:}[/latex] | [latex]\text{2H}_2\text{O} (l) + \text{2Cl}^- (aq) \rightarrow \text{H}_2 (g) + \text{Cl}_2 (g) + \text{2OH}^- (aq)[/latex] | [latex]\text{E}_\text{cell} ^\circ \text{= -2.186 V}[/latex] |
This electrolysis reaction is part of the chlor-alkali process used by industry to produce chlorine and sodium hydroxide (lye).
CHEMISTRY IN EVERYDAY LIFE
Electroplating
An important use for electrolytic cells is in electroplating. Electroplating results in a thin coating of one metal on top of a conducting surface. Reasons for electroplating include making the object more corrosion resistant, strengthening the surface, producing a more attractive finish, or for purifying metal. The metals commonly used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. Common consumer products include silver-plated or gold-plated tableware, chrome-plated automobile parts, and jewelry. The silver plating of eating utensils is used here to illustrate the process.
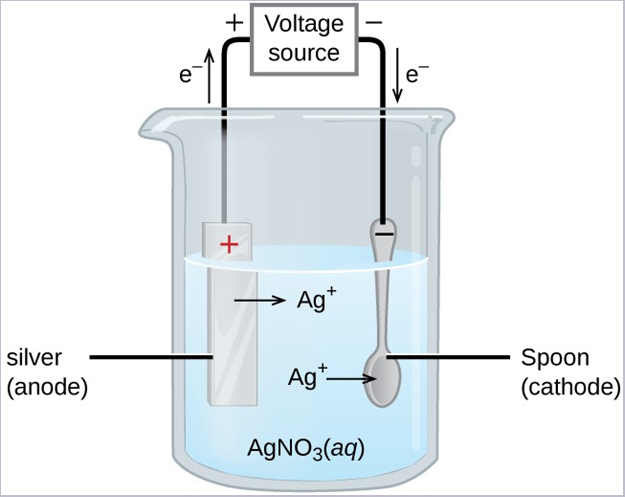
In the figure, the anode consists of a silver electrode, shown on the left. The cathode is located on the right and is the spoon, which is made from inexpensive metal. Both electrodes are immersed in a solution of silver nitrate. Applying a sufficient potential results in the oxidation of the silver anode
[latex]\text{anode: Ag} (s) \rightarrow \text{Ag}^+ (aq) + \text{e}^-[/latex]
and reduction of silver ion at the (spoon) cathode:
[latex]\text{cathode: Ag}^+ (aq) + \text{e}^-\rightarrow \text{Ag} (s)[/latex]
The net result is the transfer of silver metal from the anode to the cathode. Several experimental factors must be carefully controlled to obtain high-quality silver coatings, including the exact composition of the electrolyte solution, the cell voltage applied, and the rate of the electrolysis reaction (electrical current).
KEY TAKEAWAYS
Nonspontaneous redox processes may be forced to occur in electrochemical cells by the application of an appropriate potential using an external power source—a process known as electrolysis. Electrolysis is the basis for certain ore refining processes, the industrial production of many chemical commodities, and the electroplating of metal coatings on various products.
Chemistry End of Chapter Exercises
- Write the half-reactions and cell reaction occurring during electrolysis of each molten salt below. (a) [latex]\text{CaCl}_2[/latex] (b) [latex]\text{LiH}[/latex] (c) [latex]\text{AlCl}_3[/latex] (d) [latex]\text{CrBr}_3[/latex]
Glossary
- electrolysis
- process using electrical energy to cause a nonspontaneous process to occur
- electrolytic cell
- electrochemical cell in which an external source of electrical power is used to drive an otherwise nonspontaneous process
This chapter is an adaptation of the chapter “Electrolysis” in Chemistry: Atoms First 2e by OpenStax and is licensed under a CC BY 4.0 license.
Access for free at https://openstax.org/books/chemistry-atoms-first-2e/pages/1-introduction
process using electrical energy to cause a nonspontaneous process to occur
element that conducts heat and electricity moderately well, and possesses some properties of metals and some properties of nonmetals
element that is shiny, malleable, good conductor of heat and electricity

