83 Carbohydrates
LumenLearning
Carbohydrate Molecules
Carbohydrates are essential macromolecules that are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
LEARNING OBJECTIVES
Describe the structure of mono-, di-, and poly-saccharides
KEY TAKEAWAYS
Key Points
- Monosaccharides are simple sugars made up of three to seven carbons, and they can exist as a linear chain or as ring-shaped molecules.
- Glucose, galactose, and fructose are monosaccharide isomers, which means they all have the same chemical formula but differ structurally and chemically.
- Disaccharides form when two monosaccharides undergo a dehydration reaction (a condensation reaction); they are held together by a covalent bond.
- Sucrose (table sugar) is the most common disaccharide, which is composed of the monomers glucose and fructose.
- A polysaccharide is a long chain of monosaccharides linked by glycosidic bonds; the chain may be branched or unbranched and can contain many types of monosaccharides.
Key Terms
- isomer: Any of two or more compounds with the same molecular formula but with different structure.
- dehydration reaction: A chemical reaction in which two molecules are covalently linked in a reaction that generates [latex]\text{H}_2\text{O}[/latex] as a second product.
- biopolymer: Any macromolecule of a living organism that is formed from the polymerization of smaller entities; a polymer that occurs in a living organism or results from life.
Carbohydrates can be represented by the stoichiometric formula [latex]( \text{CH}_2\text{O})_\text{n}[/latex], where n is the number of carbons in the molecule. Therefore, the ratio of carbon to hydrogen to oxygen is 1:2:1 in carbohydrate molecules. The origin of the term “carbohydrate” is based on its components: carbon (“carbo”) and water (“hydrate”). Carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars. In monosaccharides, the number of carbons usually ranges from three to seven. If the sugar has an aldehyde group (the functional group with the structure [latex]\text{R-CHO}[/latex]), it is known as an aldose, and if it has a ketone group (the functional group with the structure [latex]\text{RC(=O)R’}[/latex]), it is known as a ketose. Depending on the number of carbons in the sugar, they also may be known as trioses (three carbons), pentoses (five carbons), and or hexoses (six carbons). Monosaccharides can exist as a linear chain or as ring-shaped molecules; in aqueous solutions they are usually found in ring forms.
Common Monosaccharides
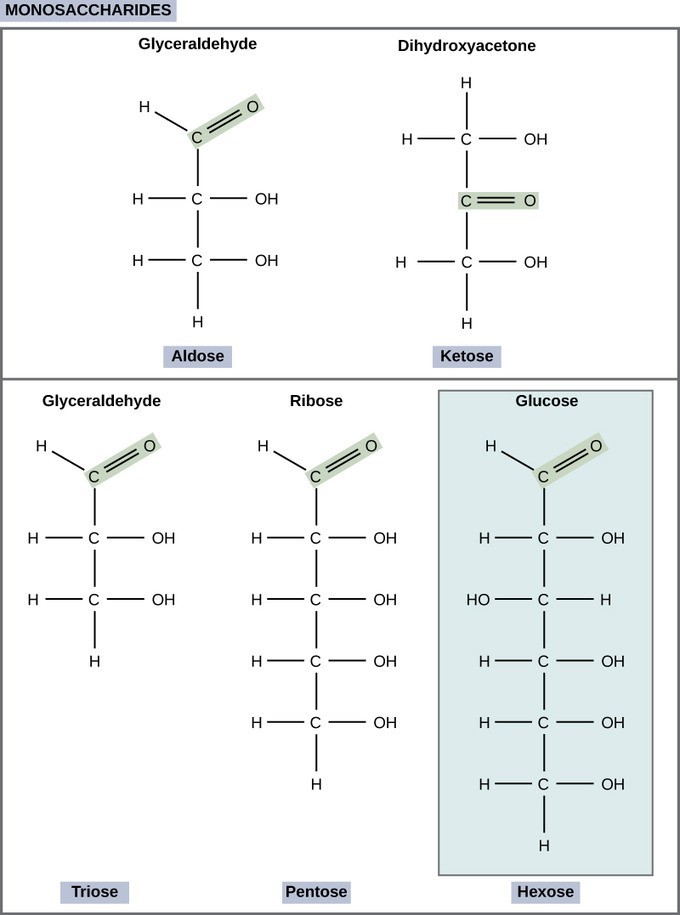
Glucose ([latex]\text{C}_6\text{H}_{12}\text{O}_6[/latex]) is a common monosaccharide and an important source of energy. During cellular respiration, energy is released from glucose and that energy is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water, and glucose, in turn, is used for energy requirements for the plant.
Galactose (a milk sugar) and fructose (found in fruit) are other common monosaccharides. Although glucose, galactose, and fructose all have the same chemical formula ([latex]\text{C}_6\text{H}_{12}\text{O}_6[/latex]), they differ structurally and stereochemically. This makes them different molecules despite sharing the same atoms in the same proportions, and they are all isomers of one another, or isomeric monosaccharides. Glucose and galactose are aldoses, and fructose is a ketose.
Disaccharides
Disaccharides (di- = “two”) form when two monosaccharides undergo a dehydration reaction (also known as a condensation reaction or dehydration synthesis). During this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of water and forming a covalent bond. A covalent bond formed between a carbohydrate molecule and another molecule (in this case, between two monosaccharides) is known as a glycosidic bond. Glycosidic bonds (also called glycosidic linkages) can be of the alpha or the beta type.
Common Disaccharides
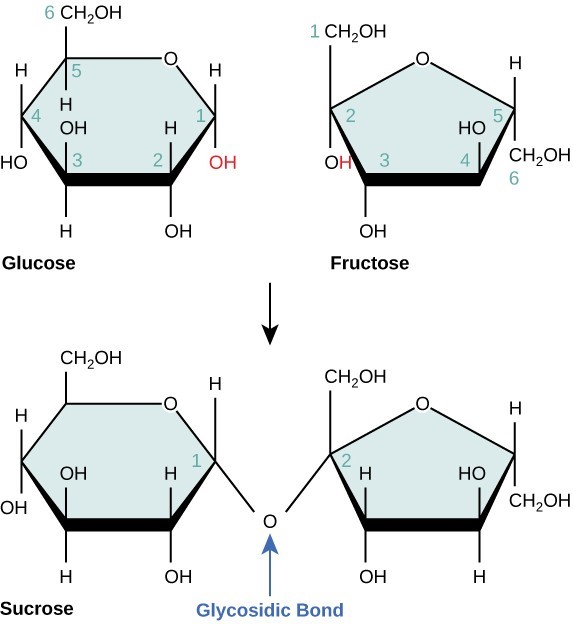
Common disaccharides include lactose, maltose, and sucrose. Lactose is a disaccharide consisting of the monomers glucose and galactose. It is found naturally in milk. Maltose, or malt sugar, is a disaccharide formed by a dehydration reaction between two glucose molecules. The most common disaccharide is sucrose, or table sugar, which is composed of the monomers glucose and fructose.
Polysaccharides
A long chain of monosaccharides linked by glycosidic bonds is known as a polysaccharide (poly- = “many”). The chain may be branched or unbranched, and it may contain different types of monosaccharides. Starch, glycogen, cellulose, and chitin are primary examples of polysaccharides.
Plants are able to synthesize glucose, and the excess glucose is stored as starch in different plant parts, including roots and seeds. Starch is the stored form of sugars in plants and is made up of glucose monomers that are joined by α1-4 or 1-6 glycosidic bonds. The starch in the seeds provides food for the embryo as it germinates while the starch that is consumed by humans is broken down by enzymes into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose.
Common Polysaccharides
Glycogen is the storage form of glucose in humans and other vertebrates. It is made up of monomers of glucose. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in liver and muscle cells. Whenever blood glucose levels decrease, glycogen is broken down to release glucose in a process known as glycogenolysis.
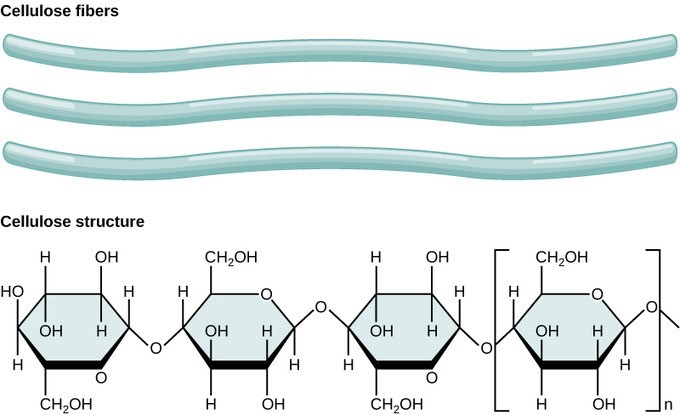
Cellulose is the most abundant natural biopolymer. The cell wall of plants is mostly made of cellulose and provides structural support to the cell. Cellulose is made up of glucose monomers that are linked by β 1-4 glycosidic bonds. Every other glucose monomer in cellulose is flipped over, and the monomers are packed tightly as extended long chains. This gives cellulose its rigidity and high tensile strength—which is so important to plant cells.
Carbohydrate Function
Carbohydrates serve various functions in different animals. Arthropods have an outer skeleton, the exoskeleton, which protects their internal body parts. This exoskeleton is made of chitin, which is a polysaccharide-containing nitrogen. It is made of repeating units of N-acetyl-β-d-glucosamine, a modified sugar. Chitin is also a major component of fungal cell walls.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- dehydration reaction. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/dehydration_reaction. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44400/latest/?collection=col11448/latest. License: CC BY: Attribution
- isomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/isomer. License: CC BY-SA: Attribution-ShareAlike
- biopolymer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/biopolymer. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Carbohydrates. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44400/latest/Figure_03_02_07.jpg. License: CC BY: Attribution
- OpenStax College, Carbohydrates. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44400/latest/Figure_03_02_04.jpg. License: CC BY: Attribution
- OpenStax College, Carbohydrates. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44400/latest/Figure_03_02_01.jpg. License: CC BY: Attribution
This chapter is an adaptation of the chapter "Carbohydrates" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
Molecules with the same number of atoms in different geometric arrangements.
an elimination (condensation) reaction in which the small molecule that is removed is water

