77 Alkenes and Alkynes
LumenLearning
Naming Alkenes and Alkynes
Alkenes and alkynes are named similarly to alkanes, based on the longest chain that contains the double or triple bond.
LEARNING OBJECTIVES
Translate between the structure and the name of an alkene or alkyne compound
KEY TAKEAWAYS
Key Points
- Alkenes and alkynes are named by identifying the longest chain that contains the double or triple bond.
- The chain is numbered to minimize the numbers assigned to the double or triple bond.
- The suffix of the compound is “-ene” for an alkene or “-yne” for an alkyne.
Key Terms
- Alkenes: An unsaturated hydrocarbon containing at least one carbon–carbon double bond.
- alkyne: An unsaturated hydrocarbon containing at least one carbon—carbon triple bond between two carbon atoms.
- stereoisomer: One of a set of the isomers of a compound that exhibits stereoisomerism.
Alkenes are hydrocarbons that contain one or more double bonds, while alkynes contain one or more triple bonds. The naming conventions for these compounds are similar to those for alkanes.
Identifying and Numbering the Longest Chain
Alkene and alkyne compounds are named by identifying the longest carbon chain that contains both carbons of the double or triple bond. This longest chain is named by the alkane series convention: “eth-” for two carbons; “prop-” for three carbons; “but-” for four carbons; etc. The carbon backbone is numbered from the end that yields the lowest positioning for the double or triple bond.
Adding Substituents
Substituents are added to the name as prefixes to the longest chain. Rotation is restricted around the double bond, so prefixes can be added to differentiate stereoisomers. Cis or trans is used to indicate whether higher priority substituents are located on the same or opposite sides of the bond. If the compound is cyclic, this information is noted by adding the “cyclo-” prefix.
Changing the Suffix
Next, the position of the double or triple bond is indicated using the position of the carbon in the bond with the lower backbone number, and the suffix for the compound is changed to “-ene” for an alkene and “-yne” for an alkyne. For cycloalkenes, the carbons in the double bond are numbered as positions 1 and 2.
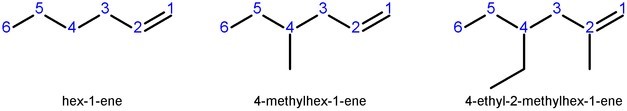
For multiple double or triple bonds, “di-,” “tri-,” or “tetra-” can be added prior to the “-ene” or “-yne.” In these cases, an extra “a” is appended to the end of the name of the alkyl chain, like in the case of butadiene. For compounds containing both double and triple bonds, the “-ene” suffix precedes the “-yne,” and the compound is numbered to minimize the bond positions.
Naming alkenes: This video shows you how to name alkene molecules using IUPAC conventions.
Properties of Alkenes
Due to the presence of a double bond in their carbon skeletons, alkenes are more reactive than their related alkanes.
LEARNING OBJECTIVES
Recognize the properties of alkenes relative to alkanes
KEY TAKEAWAYS
Key Points
- Alkenes are generally more reactive than their related alkanes due to the relative instability of the pi bond.
- The melting and boiling points of alkenes are dictated by the regularity with which they can pack and the surface area of interaction.
- Rotation is restricted around the double bond in alkenes, resulting in diastereoisomers with different substitution patterns around the double bond.
Key Terms
- diastereoisomer: A stereoisomer having multiple chiral centers; one cannot normally be superimposed on the mirror image of another.
Alkene Structures
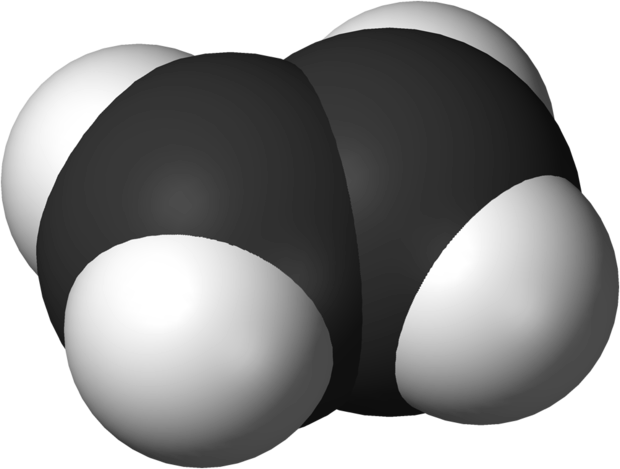
Alkenes contain a double bond that is composed of one sigma and one pi bond between two carbon atoms. The sigma bond has similar properties to those found in alkanes, while the pi bond is more reactive. The carbon atoms in the double bond are sp2 hybridized, forming a planar structure. Rotation around the double bond is disfavored, so alkenes form fairly stable isomers depending on the positioning of substituents on the same (cis) or opposite (trans) sides of the double bond. These isomers are called diastereoisomers.
Physical Properties of Alkenes

The melting and boiling points of alkenes are determined by the regularity of the packing, or the closeness, of these molecules. Alkene isomers that can achieve more regular packing have higher melting and boiling points than molecules with the same molecular formula but weaker dispersion forces. Alkenes are non-polar, and they are both immiscible in water and less dense than water. They are generally soluble in organic solvents. In addition, they do not conduct electricity.
Reactivity of Alkenes
Alkenes are more reactive than their related alkanes due to the relative instability of the double bond. They are more likely to participate in a variety of reactions, including combustion, addition, hydrogenation, and halogenation reactions. Alkenes can also be reacted, typically in the presence of a catalyst, to form polymers.
Applications
Large amounts of ethylene are produced from natural gas via thermal cracking. It is an important raw material for the synthesis of a number of plastics.
Reactions of Alkenes and Alkynes
Alkenes and alkynes are more reactive than alkanes due to their pi bonds.
LEARNING OBJECTIVES
Give examples of the various reactions that alkenes and alkynes undergo
KEY TAKEAWAYS
Key Points
- Addition reactions involving alkenes and alkynes include hydrogenation, halogenation, and hydrohalogenation.
- Alkenes and alkynes are useful reagents in polymer synthesis—an important industrial application.
- Hydrogenation reactions typically employ a metallic catalyst consisting of platinum, nickel, palladium, or rhodium.
Key Terms
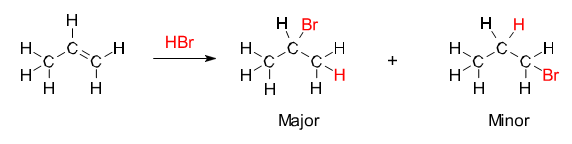
- Markovnikov’s rule: States that, with the addition of a protic acid HX to an alkene, the acid hydrogen ([latex]\text{H}[/latex]) becomes attached to the carbon with fewer alkyl substituents, and the halide (X) group becomes attached to the carbon with more alkyl substituents.
- polymer: A long or larger molecule consisting of a chain or network of many repeating units; formed by chemically bonding together many identical or similar small molecules called monomers.
Reactions of Alkenes and Alkynes
Alkenes and alkynes are generally more reactive than alkanes due to the electron density available in their pi bonds. In particular, these molecules can participate in a variety of addition reactions and can be used in polymer formation.
Addition Reactions
Unsaturated hydrocarbons can participate in a number of different addition reactions across their double or triple bonds.
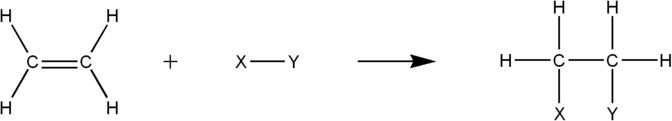
These addition reactions include catalytic hydrogenation (addition of H2), halogenation (reaction with X2, where X is a halogen ), and hydrohalogenation (reaction with H-X, where X is a halogen), among others.
Cycloaddition
Alkenes undergo diverse cycloaddition reactions. Most notable is the Diels–Alder reaction with 1,3-dienes to give cyclohexenes.
This general reaction has been extensively developed, and electrophilic alkenes and alkynes are especially effective dienophiles. Cycloaddition processes involving alkynes are often catalyzed by metals.
Oxidation
Oxidation of alkynes by strong oxidizing agents such as potassium permanganate or ozone will yield a pair of carboxylic acids. The general reaction can be pictured as:
[latex]\text{RC} \equiv \text{CR'} \xrightarrow{\text{KMnO}_4} \text{RCO}_2\text{H} + \text{R'CO}_2\text{H}[/latex]
By contrast, alkenes can be oxidized at low temperatures to form glycols. At higher temperatures, the glycol will further oxidize to yield a ketone and a carboxylic acid:
[latex]\text{(H}_3 \text{C)}_2 \text{C} = \text{CHCH}_3 \xrightarrow[\text{heat}]{\text{KMnO}_4} \text{H}_3\text{CCOCH}_3 + \text{H}_3\text{CCO}_2\text{H}[/latex]
Here, we have 3-methyl-2-butene oxidizing to form acetone and acetic acid.
Hydrogenation
In the presence of a catalyst—typically platinum, palladium, nickel, or rhodium—hydrogen can be added across a triple or a double bond to take an alkyne to an alkene or an alkene to an alkane. In practice, it is difficult to isolate the alkene product of this reaction, though a poisoned catalyst—a catalyst with fewer available reactive sites—can be used to do so. As the hydrogen is immobilized on the surface of the catalyst, the triple or double bonds are hydrogenated in a syn fashion; that is to say, the hydrogen atoms add to the same side of the molecule.
Halogenation
Alkenes and alkynes can also be halogenated with the halogen adding across the double or triple bond, in a similar fashion to hydrogenation. The halogenation of an alkene results in a dihalogenated alkane product, while the halogenation of an alkyne can produce a tetrahalogenated alkane.
Hydrohalogenation
Alkenes and alkynes can react with hydrogen halides like [latex]\text{HCl}[/latex] and [latex]\text{HBr}[/latex]. Hydrohalogenation gives the corresponding vinyl halides or alkyl dihalides, depending on the number of HX equivalents added. The addition of water to alkynes is a related reaction, except the initial enol intermediate converts to the ketone or aldehyde. If the alkene is asymmetric, the reaction will follow Markovnikov’s rule—the halide will be added to the carbon with more alkyl substituents.
Hydration
Water can be added across triple bonds in alkynes to yield aldehydes and ketones for terminal and internal alkynes, respectively. Hydration of alkenes via oxymercuration produces alcohols. This reaction takes place during the treatment of alkenes with a strong acid as the catalyst.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Alkynes. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkynes. License: CC BY-SA: Attribution-ShareAlike
- Alkenes. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkenes. License: CC BY-SA: Attribution-ShareAlike
- stereoisomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/stereoisomer. License: CC BY-SA: Attribution-ShareAlike
- alkyne. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alkyne. License: CC BY-SA: Attribution-ShareAlike
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- Naming alkenes. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Alkenes. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkenes. License: CC BY-SA: Attribution-ShareAlike
- diastereoisomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/diastereoisomer. License: CC BY-SA: Attribution-ShareAlike
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- Naming alkenes. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- Russian Cracking. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Russian_Cracking.jpg. License: Public Domain: No Known Copyright
- Alkynes. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkynes. License: CC BY-SA: Attribution-ShareAlike
- Unsaturated hydrocarbons. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Unsaturated_hydrocarbons. License: CC BY-SA: Attribution-ShareAlike
- Markovnikov’s rule. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Markovnikov’s%20rule. License: CC BY-SA: Attribution-ShareAlike
- polymer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/polymer. License: CC BY-SA: Attribution-ShareAlike
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- Naming alkenes. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- Russian Cracking. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Russian_Cracking.jpg. License: Public Domain: No Known Copyright
- Alkene. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkene. License: Public Domain: No Known Copyright
- MarkovnikovRulePropeneHBr. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:MarkovnikovRulePropeneHBr.svg. License: CC BY-SA: Attribution-ShareAlike
This chapter is an adaptation of the chapter "Alkenes and Alkynes" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
An unsaturated hydrocarbon containing at least one carbon–carbon double bond.
molecule consisting of carbon and hydrogen containing at least one carbon-carbon triple bond
One of a set of the isomers of a compound that exhibits stereoisomerism.
A stereoisomer having multiple chiral centers; one cannot normally be superimposed on the mirror image of another.
States that, with the addition of a protic acid HX to an alkene, the acid hydrogen (H) becomes attached to the carbon with fewer alkyl substituents, and the halide (X) group becomes attached to the carbon with more alkyl substituents.
A long or larger molecule consisting of a chain or network of many repeating units; formed by chemically bonding together many identical or similar small molecules called monomers.

