35 Nuclear Reactions
LumenLearning
Balancing Nuclear Equations
To balance a nuclear equation, the mass number and atomic numbers of all particles on either side of the arrow must be equal.
LEARNING OBJECTIVES
Produce a balanced nuclear equation
KEY TAKEAWAYS
Key Points
- A balanced nuclear equation is one where the sum of the mass numbers (the top number in notation) and the sum of the atomic numbers balance on either side of an equation.
- Nuclear equation problems will often be given such that one particle is missing.
- Instead of using the full equations, in many situations a compact notation is used to describe nuclear reactions.
Nuclear reactions may be shown in a form similar to chemical equations, for which invariant mass, which is the mass not considering the mass defect, must balance for each side of the equation. The transformations of particles must follow certain conservation laws, such as conservation of charge and baryon number, which is the total atomic mass number. An example of this notation follows:
[latex]_3^6 \text{Li} + \ _1^2 \text{H} \rightarrow _2^4 \text{He} + \text{?}[/latex]
To balance the equation above for mass, charge, and mass number, the second nucleus on the right side must have atomic number 2 and mass number 4; it is therefore also helium-4. The complete equation therefore reads:
[latex]_3^6 \text{Li} + \ _1^2 \text{H} \rightarrow _2^4 \text{He} + \ _2^4 \text{He}[/latex]
Or, more simply:
[latex]_3^6 \text{Li} + \ _1^2 \text{H} \rightarrow 2 _2^4 \text{He}[/latex]
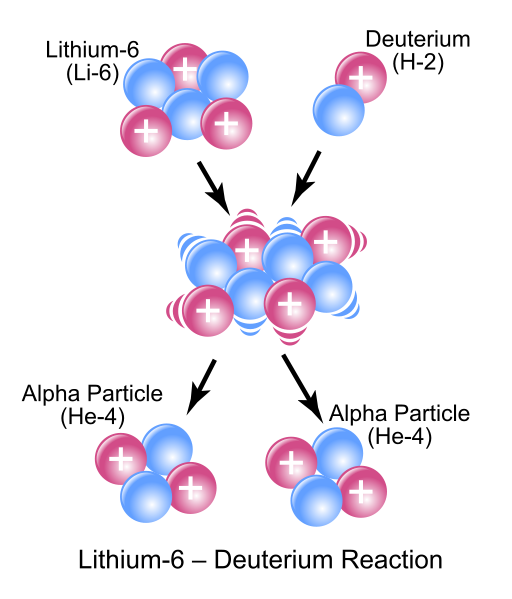
Balancing a Radioactive Decay Equation
In balancing a nuclear equation, it is important to remember that the sum of all the mass numbers and atomic numbers, given on the upper left and lower left side of the element symbol, respectively, must be equal for both sides of the equation. In addition, problems will also often be given as word problems, so it is useful to know the various names of radioactively emitted particles.
EXAMPLE
[latex]_{92}^{235}\text{U} \rightarrow \ _{90}^{231}\text{Th} + \text{?}[/latex]
This could be written out as uranium-235 gives thorium-231 plus what? In order to solve, we find the difference between the atomic masses and atomic numbers in the reactant and product. The result is an atomic mass difference of 4 and an atomic number difference of 2. This fits the description of an alpha particle. Thus, we arrive at our answer:
[latex]_{92}^{235} \text{U} \rightarrow \ _{90}^{231} \text{Th} + \ _2^4 \text{He}[/latex]
EXAMPLE
[latex]_{84}^{214} \text{Po} + 2 _2^4 \text{He} + 2 _{-1}^{\ 0} e \rightarrow \text{?}[/latex]
This could also be written out as polonium-214, plus two alpha particles, plus two electrons, give what? In order to solve this equation, we simply add the mass numbers, 214 for polonium, plus 8 (two times four) for helium (two alpha particles), plus zero for the electrons, to give a mass number of 222. For the atomic number, we take 84 for polonium, add 4 (two times two) for helium, then subtract two (two times -1) for two electrons lost through beta emission, to give 86; this is the atomic number for radon ([latex]\text{Rn}[/latex]). Therefore, the equation should read:
[latex]_{84}^{214} \text{Po} + 2 _2^4 \text{He} + 2 _{-1}^0 e \rightarrow \ _{86}^{222} \text{Rn}[/latex]
Writing nuclear equations: Describes how to write the nuclear equations for alpha and beta decay.
Nuclear Binding Energy and Mass Defect
A nucleus weighs less than its sum of nucleons, a quantity known as the mass defect, caused by release of energy when the nucleus formed.
LEARNING OBJECTIVES
Calculate the mass defect and nuclear binding energy of an atom
KEY TAKEAWAYS
Key Points
- Nuclear binding energy is the energy required to split a nucleus of an atom into its components.
- Nuclear binding energy is used to determine whether fission or fusion will be a favorable process.
- The mass defect of a nucleus represents the mass of the energy binding the nucleus, and is the difference between the mass of a nucleus and the sum of the masses of the nucleons of which it is composed.
Key Terms
- nucleon: One of the subatomic particles of the atomic nucleus, i.e. a proton or a neutron.
- strong force: The nuclear force, a residual force responsible for the interactions between nucleons, deriving from the color force.
- mass defect: The difference between the calculated mass of the unbound system and the experimentally measured mass of the nucleus.
Binding Energy
Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts: protons and neutrons, or, collectively, the nucleons. The binding energy of nuclei is always a positive number, since all nuclei require net energy to separate them into individual protons and neutrons.
Mass Defect
Nuclear binding energy accounts for a noticeable difference between the actual mass of an atom’s nucleus and its expected mass based on the sum of the masses of its non-bound components.
Recall that energy (E) and mass (m) are related by the equation:
[latex]\text{E} = \text{mc}^2[/latex]
Here, c is the speed of light. In the case of nuclei, the binding energy is so great that it accounts for a significant amount of mass.
The actual mass is always less than the sum of the individual masses of the constituent protons and neutrons because energy is removed when when the nucleus is formed. This energy has mass, which is removed from the total mass of the original particles. This mass, known as the mass defect, is missing in the resulting nucleus and represents the energy released when the nucleus is formed.
Mass defect (Md) can be calculated as the difference between observed atomic mass (mo) and that expected from the combined masses of its protons (mp, each proton having a mass of 1.00728 amu) and neutrons (mn, 1.00867 amu):
[latex]\text{M}_\text{d} = (\text{m}_\text{n} + \text{m}_\text{p}) - \text{m}_\text{o}[/latex]
Nuclear Binding Energy
Once mass defect is known, nuclear binding energy can be calculated by converting that mass to energy by using E=mc2. Mass must be in units of kg.
Once this energy, which is a quantity of joules for one nucleus, is known, it can be scaled into per-nucleon and per- mole quantities. To convert to joules/mole, simply multiply by Avogadro’s number. To convert to joules per nucleon, simply divide by the number of nucleons.
Nuclear binding energy can also apply to situations when the nucleus splits into fragments composed of more than one nucleon; in these cases, the binding energies for the fragments, as compared to the whole, may be either positive or negative, depending on where the parent nucleus and the daughter fragments fall on the nuclear binding energy curve. If new binding energy is available when light nuclei fuse, or when heavy nuclei split, either of these processes result in the release of the binding energy. This energy—available as nuclear energy—can be used to produce nuclear power or build nuclear weapons. When a large nucleus splits into pieces, excess energy is emitted as photons, or gamma rays, and as kinetic energy, as a number of different particles are ejected.
Nuclear binding energy is also used to determine whether fission or fusion will be a favorable process. For elements lighter than iron-56, fusion will release energy because the nuclear binding energy increases with increasing mass. Elements heavier than iron-56 will generally release energy upon fission, as the lighter elements produced contain greater nuclear binding energy. As such, there is a peak at iron-56 on the nuclear binding energy curve.
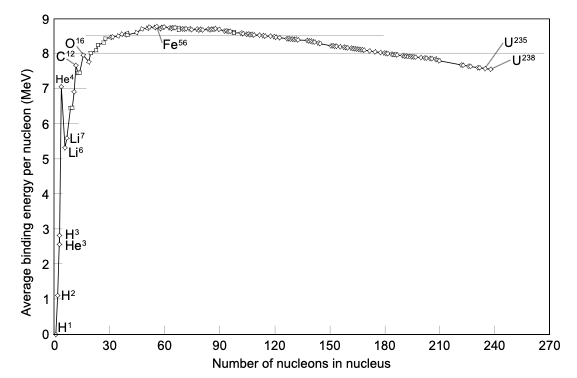
The rationale for this peak in binding energy is the interplay between the coulombic repulsion of the protons in the nucleus, because like charges repel each other, and the strong nuclear force, or strong force. The strong force is what holds protons and neutrons together at short distances. As the size of the nucleus increases, the strong nuclear force is only felt between nucleons that are close together, while the coulombic repulsion continues to be felt throughout the nucleus; this leads to instability and hence the radioactivity and fissile nature of the heavier elements.
EXAMPLE
Calculate the average binding energy per mole of a U-235 isotope. Show your answer in kJ/mole.
First, you must calculate the mass defect. U-235 has 92 protons, 143 neutrons, and has an observed mass of 235.04393 amu.
[latex]\text{M}_\text{d} = (\text{m}_\text{n} + \text{m}_\text{p}) - \text{m}_\text{o}[/latex]
[latex]\text{M}_\text{d} = (92(1.00728 \text{ amu}) + 143(1.00867 \text{ amu})) – 235.04393 \text{ amu}[/latex]
[latex]\text{M}_\text{d} = 1.86564 \text{ amu}[/latex]
Calculate the mass in kg:
[latex]1.86564 \text{ amu } \times \frac{\text{1 kg}}{6.02214 \times 10^{26} \text{ amu }} = 3.09797 \times 10^{-27} \text{ kg}[/latex]
Now calculate the energy:
[latex]E = mc^2[/latex]
[latex]E = 3.09797 \times 10^{-27} \text{ kg} \times (2.99792458 \times 10^8 \frac{\text{m}}{\text{s}})^2[/latex]
[latex]E = 2.7843 \times 10^{-10} J[/latex]
Now convert to kJ per mole:
[latex]2.7843 \times 10^{-10} \frac{\text{joules}}{\text{atom}} \times \frac{6.02 \times 10^23 \text{ atoms}}{\text{mole}} \times \frac{\text{1 kJ}}{\text{1000 joules}} = 1.6762 \times 10^{11} \frac{\text{kJ}}{\text{mole}}[/latex]
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Nuclear reaction. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Nuclear_reaction. License: CC BY-SA: Attribution-ShareAlike
- baryon. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/baryon. License: CC BY-SA: Attribution-ShareAlike
- File:Li6-D%20Reaction.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Li6-D_Reaction.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Writing nuclear equations. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- Nuclear binding energy. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Nuclear_binding_energy. License: CC BY-SA: Attribution-ShareAlike
- mass defect. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/mass%20defect. License: CC BY-SA: Attribution-ShareAlike
- nucleon. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/nucleon. License: CC BY-SA: Attribution-ShareAlike
- strong force. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/strong_force. License: CC BY-SA: Attribution-ShareAlike
- File:Li6-D%20Reaction.svg%20-%20Wikipedia,%20the%20free%20encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Li6-D_Reaction.svg&page=1. License: CC BY-SA: Attribution-ShareAlike
- Writing nuclear equations. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- File:Binding energy curve – common isotopes.svg – Wikipedia, the free encyclopedia. Provided by: Wikipedia. Located at: http://en.wikipedia.org/w/index.php?title=File:Binding_energy_curve_-_common_isotopes.svg&page=1. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter "Nuclear Reactions" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
collective term for protons and neutrons in a nucleus
difference between the mass of an atom and the summed mass of its constituent subatomic particles (or the mass “lost” when nucleons are brought together to form a nucleus)

