87 Classes of Organic Compounds
LumenLearning
Organic Molecules and Functional Groups
Functional groups are groups of molecules attached to organic molecules and give them specific identities or functions.
LEARNING OBJECTIVES
Describe the importance of functional groups to organic molecules
KEY TAKEAWAYS
Key Points
- Functional groups are collections of atoms that attach the carbon skeleton of an organic molecule and confer specific properties.
- Each type of organic molecule has its own specific type of functional group.
- Functional groups in biological molecules play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
- Functional groups include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl.
Key Terms
- hydrophobic: lacking an affinity for water; unable to absorb, or be wetted by water
- hydrophilic: having an affinity for water; able to absorb, or be wetted by water
Location of Functional Groups
Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules. When functional groups are shown, the organic molecule is sometimes denoted as “R.” Functional groups are found along the “carbon backbone” of macromolecules which is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
Properties of Functional Groups
A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
Classifying Functional Groups
Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity. An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions ([latex]\text{H}^+[/latex]) from the [latex]\text{COOH}[/latex] group resulting in the negatively charged [latex]\text{COO}^-[/latex] group; this contributes to the hydrophilic nature of whatever molecule it is found on. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
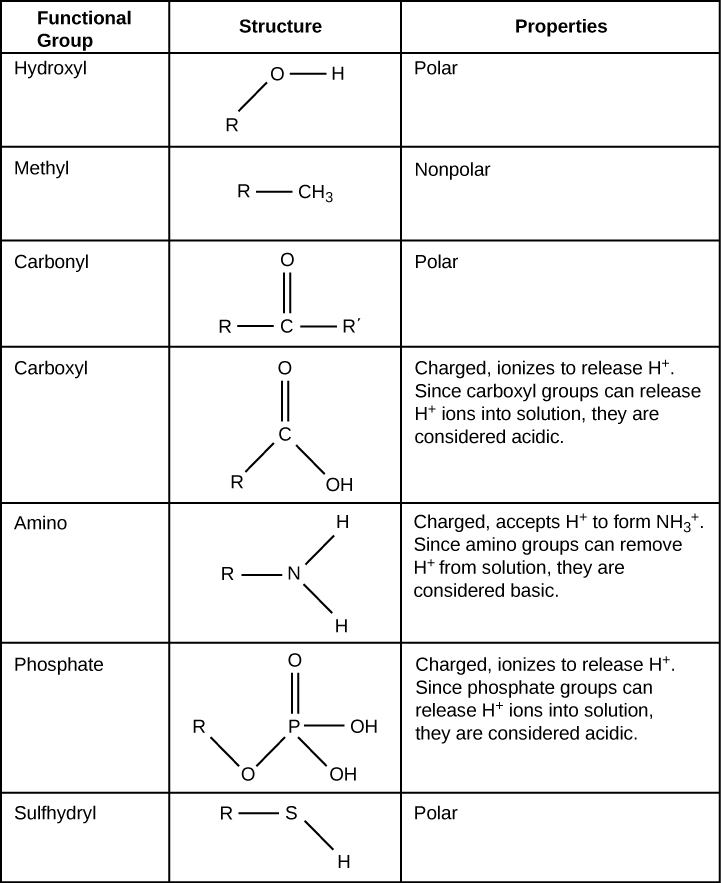
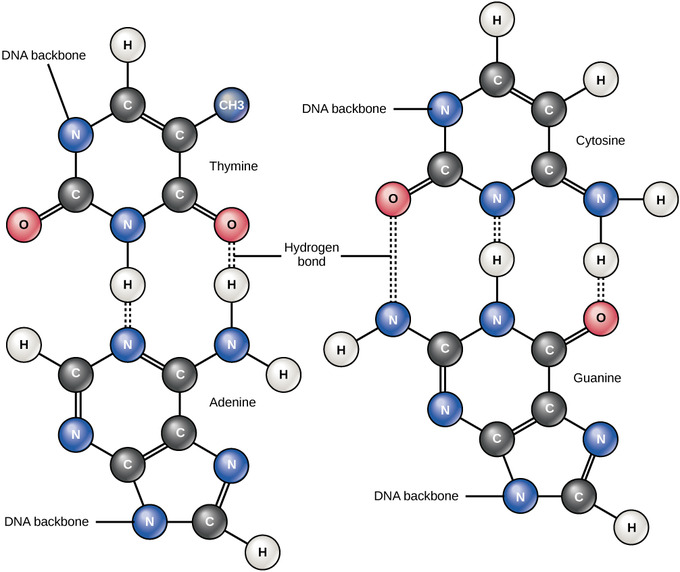
Hydrogen Bonds between Functional Groups
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly and maintain the appropriate shape needed to function correctly. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate.
The Chemical Basis for Life
Carbon is the most important element to living things because it can form many different kinds of bonds and form essential compounds.
LEARNING OBJECTIVES
Explain the properties of carbon that allow it to serve as a building block for biomolecules
KEY TAKEAWAYS
Key Points
- All living things contain carbon in some form.
- Carbon is the primary component of macromolecules, including proteins, lipids, nucleic acids, and carbohydrates.
- Carbon’s molecular structure allows it to bond in many different ways and with many different elements.
- The carbon cycle shows how carbon moves through the living and non-living parts of the environment.
Key Terms
- octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons (has some exceptions).
- carbon cycle: the physical cycle of carbon through the earth’s biosphere, geosphere, hydrosphere, and atmosphere; includes such processes as photosynthesis, decomposition, respiration and carbonification
- macromolecule: a very large molecule, especially used in reference to large biological polymers (e.g., nucleic acids and proteins)
Carbon is the fourth most abundant element in the universe and is the building block of life on earth. On earth, carbon circulates through the land, ocean, and atmosphere, creating what is known as the Carbon Cycle. This global carbon cycle can be divided further into two separate cycles: the geological carbon cycles takes place over millions of years, whereas the biological or physical carbon cycle takes place from days to thousands of years. In a nonliving environment, carbon can exist as carbon dioxide ([latex]\text{CO}_2[/latex]), carbonate rocks, coal, petroleum, natural gas, and dead organic matter. Plants and algae convert carbon dioxide to organic matter through the process of photosynthesis, the energy of light.

Carbon is Important to Life
In its metabolism of food and respiration, an animal consumes glucose ([latex]\text{C}_6\text{H}_{12}\text{O}_6[/latex]), which combines with oxygen ([latex]\text{O}_2[/latex]) to produce carbon dioxide ([latex]\text{CO}_2[/latex]), water ([latex]\text{H}_2\text{O}[/latex]), and energy, which is given off as heat. The animal has no need for the carbon dioxide and releases it into the atmosphere. A plant, on the other hand, uses the opposite reaction of an animal through photosynthesis. It intakes carbon dioxide, water, and energy from sunlight to make its own glucose and oxygen gas. The glucose is used for chemical energy, which the plant metabolizes in a similar way to an animal. The plant then emits the remaining oxygen into the environment.
Cells are made of many complex molecules called macromolecules, which include proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
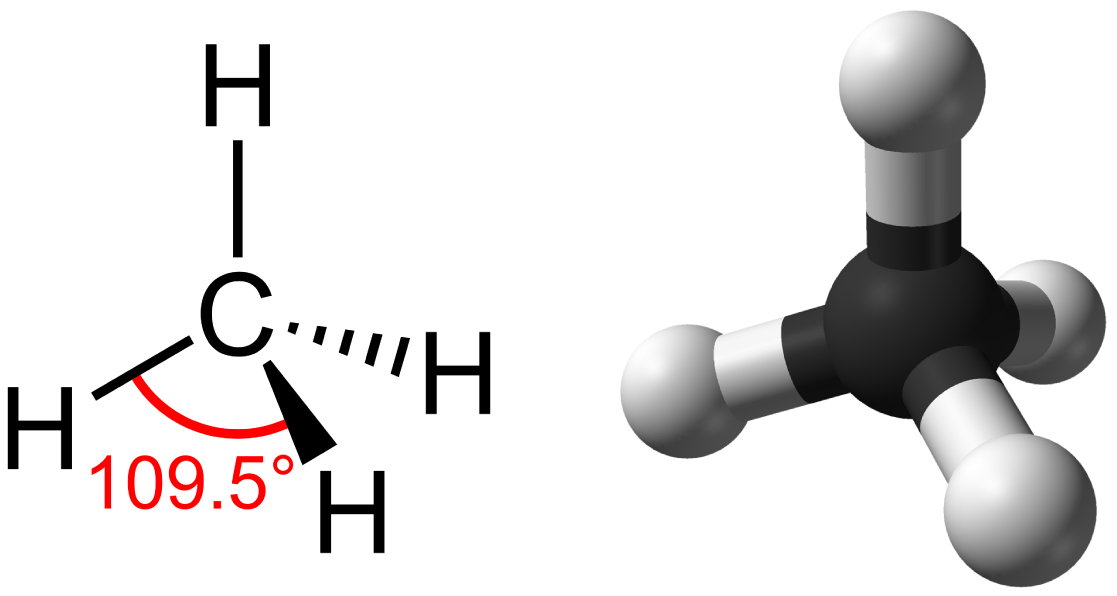
Structure of Carbon
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula [latex]\text{CH}_4[/latex]. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/?collection=col11448/latest. License: CC BY: Attribution
- hydrophilic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/hydrophilic. License: CC BY-SA: Attribution-ShareAlike
- hydrophobic. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/hydrophobic. License: CC BY-SA: Attribution-ShareAlike
- Functional Group. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Functional_group. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Carbon. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/Figure_02_03_08.jpg. License: CC BY: Attribution
- OpenStax College, Carbon. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/Figure_02_03_07.jpg. License: CC BY: Attribution
- octet rule. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/octet_rule. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/?collection=col11448/latest. License: CC BY: Attribution
- The Carbon Cycle. Provided by: climate-jigsaw Wikispace. Located at: http://climate-jigsaw.wikispaces.com/The+Carbon+Cycle. License: CC BY-SA: Attribution-ShareAlike
- macromolecule. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/macromolecule. License: CC BY-SA: Attribution-ShareAlike
- carbon cycle. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/carbon_cycle. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Carbon. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/Figure_02_03_08.jpg. License: CC BY: Attribution
- OpenStax College, Carbon. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/Figure_02_03_07.jpg. License: CC BY: Attribution
- OpenStax College, Carbon. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44393/latest/Figure_02_03_01f.jpg. License: CC BY: Attribution
- A leaf with laminar structure andu00a0pinnateu00a0venation. Provided by: Wikimedia Commons. Located at: http://en.wikipedia.org/wiki/Leaf%23mediaviewer/File:Leaf_1_web.jpg. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter "Classes of Organic Compounds" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
guideline that states main group atoms will form structures in which eight valence electrons interact with each nucleus, counting bonding electrons as interacting with both atoms connected by the bond

