75 Aliphatic Hydrocarbons
LumenLearning
Alkanes
Alkanes are relatively unreactive hydrocarbons that contain no double or triple bonds in their carbon skeletons.
LEARNING OBJECTIVES
Identify the basic properties of alkanes
KEY TAKEAWAYS
Key Points
- Alkanes are saturated hydrocarbons, which means that their carbon backbones contain no double or triple bonds.
- Due to the valence configuration of carbon, there are a variety of alkane isomers, which are commonly separated into linear and branched structures.
- Alkanes are used in a number of industrial applications and are found in natural gas and petroleum.
Key Terms
- saturated hydrocarbon: A compound consisting entirely of hydrogen and carbon, composed entirely of single bonds.
- hydrocarbon: A compound consisting only of carbon and hydrogen atoms.
Alkanes, also called paraffins, are a class of hydrocarbons that are fully saturated with hydrogen. They contain no double or triple bonds in their carbon skeletons and, therefore, have the maximum number of carbon to hydrogen covalent bonds. This is in contrast to alkenes and alkynes, which contain double and triple bonds and are known as unsaturated hydrocarbons.
Structure of Alkanes
Alkanes have the general formula [latex]\text{C}_\text{n} \text{H}_\text{2n+2}[/latex]. For example, an alkane with 2 (n) carbon atoms, will have 6 (2n + 2) hydrogen atoms. Their adjacent atoms are connected with sigma bonds and form tetrahedral centers around the carbon atoms. As these bonds are all single bonds, there is free rotation around all connections. Each carbon atom has four bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane (e.g., C2-alkane).
An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms; for example, R could represent a methyl or ethyl group. An alkyl group is a piece of a molecule with the general formula [latex]( \text{CH}_3 )_\text{n}[/latex], where n is any integer. For example, a methyl group [latex]( \text{CH}_3 )[/latex] is a fragment of a methane molecule [latex]( \text{CH}_4 )[/latex]. In this example, n=1.
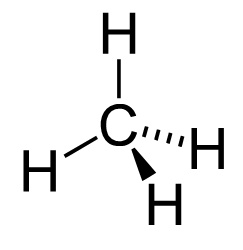
The simplest possible alkane is methane [latex]( \text{CH}_4 )[/latex]. Saturated oils and waxes are examples of larger alkanes where the number of carbons in the carbon backbone is greater than ten.
In linear alkanes, the carbon atoms are joined in a snake-like structure. In branched alkanes, the carbon backbone splits off in one or more directions. In cyclic alkanes, the carbon backbone is linked so as to form a loop. Cyclic and branched alkanes will be discussed in greater detail in subsequent sections.
Nomenclature for Alkanes
Alkanes are named with the suffix “-ane” following the hydrocarbon prefixes. The series contains methane [latex]( \text{CH}_4 )[/latex], ethane [latex]( \text{C}_2\text{H}_6 )[/latex], propane [latex]( \text{C}_3\text{H}_8 )[/latex], butane [latex]( \text{C}_4\text{H}_{10} )[/latex], pentane [latex]( \text{C}_5\text{H}_{12} )[/latex], and so on. For carbon chains with length of 6, 7, 8, 9, and 10 atoms, the prefixes are “hex-,” “hept-,” “oct-,” “non-,” and “dec-,” respectively.
For the higher molecular weight compounds, the four bonds formed by carbon allow for a number of variations on the carbon skeleton. These multiple forms, which share the same molecular formula, are known as isomers. The prefix “n-,” for normal, is reserved for the linear, unbranched forms of these alkanes.
Properties of Alkanes
The smaller members of the alkane family are gases, while the larger compounds are liquid and solid compounds. They are commonly found in fuel sources, like natural gas and petroleum. The solid compounds are typically waxy in texture.
Alkanes have a number of industrial applications beyond fuels, including uses in cosmetics and plastics. Alkanes are generally less reactive than alkenes and alkynes because they lack the more reactive double and triple bonds. However, they do participate in reactions with oxygen (combustion) and halogens.
Reactions of Alkanes
Alkanes are generally unreactive, but can participate in oxidation, halogenation, and cracking reactions.
LEARNING OBJECTIVES
Identify the general reactions of alkanes
KEY TAKEAWAYS
Key Points
- Alkanes are stable compounds and are generally unreactive.
- The most important application of alkanes is in oxidation reactions; they are used in internal combustion engines as fuel.
- Applying heat and a catalyst can crack larger, more complex alkanes and produce smaller, more useful alkanes and alkenes.
Key Terms
- cracking: The thermal decomposition of a substance, especially that of crude petroleum, in order to produce petrol/gasoline.
- radical: A group of atoms joined by covalent bonds that are characterized by a free, unpaired electron that imparts their reactivity.
- alkane: Any of the saturated hydrocarbons (including methane, ethane, and compounds with long carbon chain known as paraffins, etc.) that have a chemical formula of the form [latex]\text{C}_\text{n} \text{H}_\text{2n+2}[/latex].
In comparison to alkenes and alkynes, alkanes are relatively unreactive due to the absence of a weaker pi bond in their carbon skeletons. However, there are a few classes of reactions that are commonly performed with alkanes.
Oxidation Reactions
The most important reaction that alkanes undergo is combustion. Smaller, linear alkanes generally oxidize more readily than larger, more branched molecules. Alkanes can be burned in the presence of oxygen to produce carbon dioxide, water, and energy; in situations with limited oxygen, the products are carbon monoxide, water, and energy. For this reason, alkanes are frequently used as fuel sources. The combustion of methane is shown:
[latex]\text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O} +\ \text{energy}[/latex]
Halogenation
With the addition of a halogen gas and energy, alkanes can be halogenated with the reactivity of the halogens proceeding in the following order: [latex]\text{Cl}_2 > \text{Br}_2 > \text{I}_2[/latex].
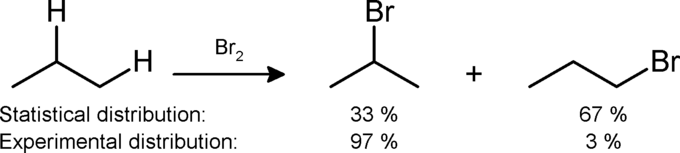
In this reaction, UV light or heat initiates a chain reaction, cleaving the covalent bond between the two atoms of a diatomic halogen. The halogen radicals can then abstract protons from the alkanes, which can then combine or react to form more radicals. Alkanes can be halogenated at a number of sites, and this reaction typically yields a mixture of halogenated products.

Thermal Cracking
The complex alkanes with high molecular weights that are found in crude oil are frequently broken into smaller, more useful alkanes by thermal cracking; alkenes and hydrogen gas are also produced by using this method. Thermal cracking is typically performed at high temperatures, and often in the presence of a catalyst. A mixture of products results, and these alkanes and alkenes can be separated by fractional distillation.
Drawing Hydrocarbon Structures
Hydrocarbon structures can be drawn from the IUPAC names of chemical compounds by starting with the carbon backbone and adding substituents.
LEARNING OBJECTIVES
Identify the structure of a hydrocarbon from its IUPAC name
KEY TAKEAWAYS
Key Points
- Hydrocarbons frequently have both historical and IUPAC names; the IUPAC names can be used to draw the chemical structures.
- To draw a hydrocarbon structure, start with the carbon backbone, which can include a ring structure.
- After the carbon backbone is drawn, add the substituent groups and the double and triple bonds that are indicated by the prefixes and suffixes in the chemical name.
Key Terms
- isomer: Any of two or more compounds with the same molecular formula, but with a different structure.
Hydrocarbons can be drawn in several equally valid ways. The most common method is using the bond line formula, which is ubiquitous for its simplicity.
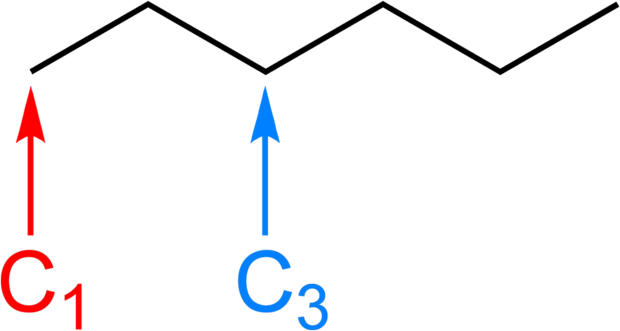
Bond Line Structures for Hydrocarbons
To draw a hydrocarbon using the bond line method, place your pencil on a piece of paper. This first dot represents one carbon atom. To continue, draw a short, straight line. Now the hydrocarbon represented by the short, straight line is two carbon atoms in length; it has two ends.
To increase the number of carbon atoms in your drawn structure, change direction and continue with a short, straight line. In addition to the two ends, there is now a vertex that represents a third carbon atom. The angle created by the three carbon atoms should be in the region of 110 degrees.
Extending the line with more and more vertices produces a longer carbon chain.
Note that no carbon atom is explicitly written; they are implicit from the number of vertices plus the two ends. Additionally, no hydrogen atoms are written. Convention assumes that every bond line structure includes enough hydrogen atoms so that all carbon atoms in the structure make four bonds. All non-hydrogen and carbon atoms should be written explicitly, however.
Drawing Carbon Skeletons
Hydrocarbon structures are drawn starting with the carbon backbone, which is the longest branch, and are named according to the
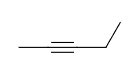
alkane series: one carbon, “meth-“; two carbons, “eth-“; three carbons, “prop-“; etc. Compounds that start with “cyclo-” are drawn starting with the ring structure.
For example, 3-ethyl-hexane could be represented by first drawing the hexane backbone, then adding an ethyl (two-carbon) chain extending from the third carbon of the backbone.
Drawing Double and Triple Bonds
Compounds ending with “-ane” consist of solely single bonds in the carbon skeleton. The presence of double or triple bonds is determined by the suffix “-ene” or “-yne,” respectively, and the positioning of the bond is defined by the numbering in the name of the structure.
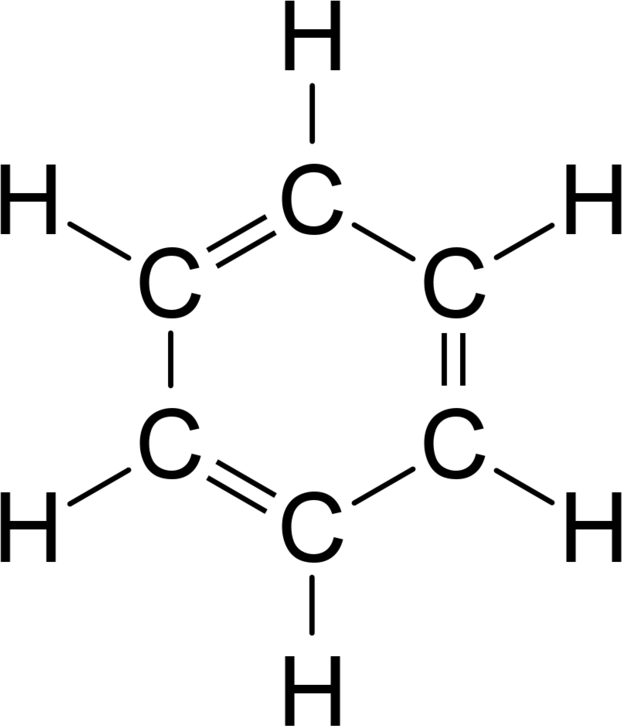
Kekulé Structures
Kekulé structures are less commonly used than bond line structures because they are more tedious to draw. The difference is that in Kekulé structures, carbon atoms are signified with C at the vertices, and hydrogen atoms bonded to carbon are explicitly drawn.
When including an alkene bond in your hydrocarbon structure, aim for 120 degree bond angles about each doubly-bonded carbon. In the case of alkyne bonds, simply draw the triple bond in-line with the carbon atoms immediately bound to the alkyne carbons.
3D Structures
The 3D configuration for substituent groups on chemical structures is often indicated by shading bonds to indicate those that are above and below the plane of the paper. These bond angles follow the sp, sp2, and sp3 hybridization patterns of the atoms in the carbon backbone.
Cycloalkanes
Cycloalkanes are saturated hydrocarbons that contain a ring in their carbon backbones.
LEARNING OBJECTIVES
Identify the general properties of cycloalkanes
KEY TAKEAWAYS
Key Points
- Cycloalkanes with one ring in their carbon backbone have the chemical formula [latex]\text{C}_\text{n} \text{H}_\text{2n}[/latex].
- Cycloalkanes are named similarly to their linear counterparts, but with the prefix “cyclo-,” such that a three carbon ring, for example, would be cyclopropane.
- The ring structure prevents cycloalkanes from achieving a tetrahedral configuration around their carbon atoms, which produces ring strain.
Key Terms
- isomer: Any of two or more compounds with the same molecular formula, but with a different structure.
General Properties of Cyclic Organic Compounds
Cycloalkanes are saturated hydrocarbons that contain a ring in their carbon backbones. Analogous ring structures containing double and triple bonds are known as cycloalkenes and cycloalkynes. Cycloalkanes with one ring have the chemical formula [latex]\text{C}_\text{n} \text{H}_\text{2n}[/latex].
Cycloalkanes, like alkanes, are subject to intermolecular forces called London dispersion forces. These weak intermolecular interactions result in relatively low melting and boiling points. Like alkanes, cycloalkanes are not particularly reactive, with the exception of the smallest, most strained cycloalkanes.
Cycloalkane Structures
Cycloalkanes are composed of sp3 hybridized carbon and hydrogen atoms connected by sigma bonds. However, unlike linear hydrocarbons, which can achieve a more stable tetrahedral configuration around each carbon atom in the backbone, the bond angles in cycloalkanes are constrained, producing ring strain.
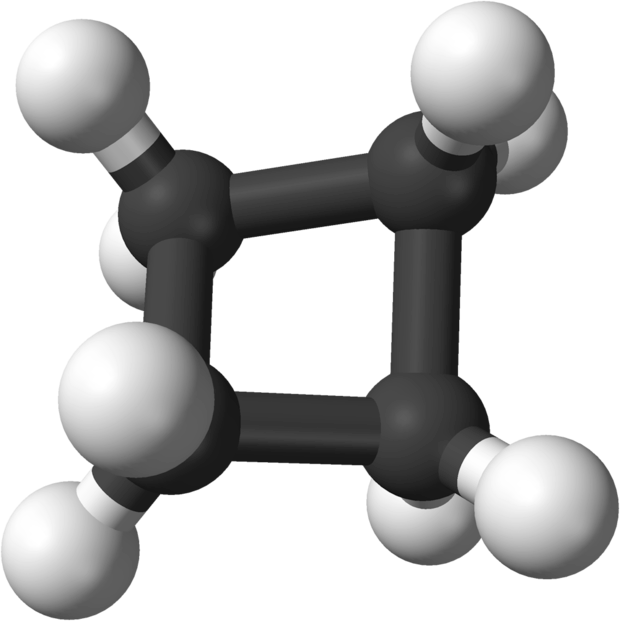
In order to achieve a more optimal configuration, the rings form puckered rather than flat structures. Cyclohexane forms a configuration called the chair configuration, along with a less stable boat configuration.
Cycloalkane Naming or Nomenclature
Cycloalkanes are named using the same conventions as linear alkanes, with the prefix cyclo- added to the front of the name. The series includes cyclopropane, cyclobutane, cyclopentane, cyclohexane, and cycloheptane.
When naming substituted cycloalkanes, the ring positions are numbered to minimize the numbering of the appended groups. Numbering is only necessary when there is more than one substituent.
Cycloalkane Isomers
The rings of cycloalkanes have two faces, and substituent groups can be appended to either face. When two substituents occupy the same face, the configuration is known as the cis isomer, while the isomer with substituents on opposite faces is referred to as the trans isomer.
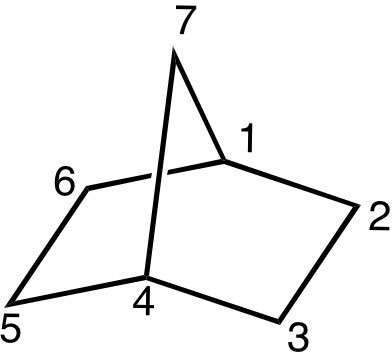
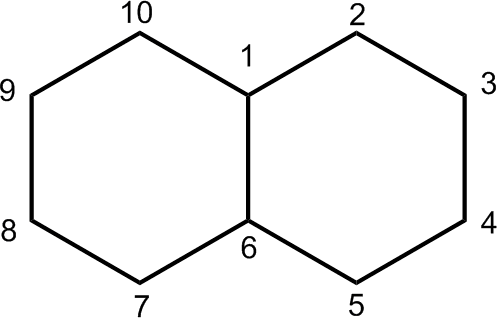
Polycyclic Compounds
Cyclic organic compounds can contain more than one ring. Hydrocarbons with two rings are called bicyclic, and well-known examples are norbornane and decalin.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision. Provided by: Boundless.com. License: CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- Alkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkane. License: CC BY-SA: Attribution-ShareAlike
- saturated hydrocarbon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/saturated%20hydrocarbon. License: CC BY-SA: Attribution-ShareAlike
- hydrocarbon. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/hydrocarbon. License: CC BY-SA: Attribution-ShareAlike
- Alkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkane. License: Public Domain: No Known Copyright
- Hexyl. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Hexyl%23Nomenclature. License: CC BY-SA: Attribution-ShareAlike
- cracking. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/cracking. License: CC BY-SA: Attribution-ShareAlike
- alkane. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/alkane. License: CC BY-SA: Attribution-ShareAlike
- radical. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/radical. License: CC BY-SA: Attribution-ShareAlike
- Alkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkane. License: Public Domain: No Known Copyright
- Cracking (chemistry). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Cracking_(chemistry). License: Public Domain: No Known Copyright
- Monobromination of propane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Monobromination_of_propane.png. License: Public Domain: No Known Copyright
- Hydrocarbon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Hydrocarbon. License: CC BY-SA: Attribution-ShareAlike
- isomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/isomer. License: CC BY-SA: Attribution-ShareAlike
- Alkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkane. License: Public Domain: No Known Copyright
- Cracking (chemistry). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Cracking_(chemistry). License: Public Domain: No Known Copyright
- Monobromination of propane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Monobromination_of_propane.png. License: Public Domain: No Known Copyright
- Benz1. Provided by: Wikimedia%20Commons. Located at: http://commons.wikimedia.org/wiki/File:Benz1.png. License: CC BY-SA: Attribution-ShareAlike
- Hydrocarbon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Hydrocarbon. License: Public Domain: No Known Copyright
- 2-pentyne. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:2-pentyne.svg. License: CC BY-SA: Attribution-ShareAlike
- Skeletal-formulae-example-1-hexane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Skeletal-formulae-example-1-hexane.png. License: CC BY-SA: Attribution-ShareAlike
- Cycloalkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Cycloalkane. License: CC BY-SA: Attribution-ShareAlike
- isomer. Provided by: Wiktionary. Located at: http://en.wiktionary.org/wiki/isomer. License: CC BY-SA: Attribution-ShareAlike
- Alkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Alkane. License: Public Domain: No Known Copyright
- Cracking (chemistry). Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Cracking_(chemistry). License: Public Domain: No Known Copyright
- Monobromination of propane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Monobromination_of_propane.png. License: Public Domain: No Known Copyright
- Benz1. Provided by: Wikimedia%20Commons. Located at: http://commons.wikimedia.org/wiki/File:Benz1.png. License: CC BY-SA: Attribution-ShareAlike
- Hydrocarbon. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Hydrocarbon. License: Public Domain: No Known Copyright
- 2-pentyne. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:2-pentyne.svg. License: CC BY-SA: Attribution-ShareAlike
- Skeletal-formulae-example-1-hexane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/File:Skeletal-formulae-example-1-hexane.png. License: CC BY-SA: Attribution-ShareAlike
- Decalin%20ssg. Provided by: Wikimedia%20commons. Located at: http://en.wikipedia.org/wiki/Decalin%23mediaviewer/File:Decahydronaphthalene.png. License: Public Domain: No Known Copyright
- NorbornaneNumbering.png. Provided by: Wikimedia%2520Commons. Located at: http://commons.wikimedia.org/wiki/File:NorbornaneNumbering.png. License: CC BY-SA: Attribution-ShareAlike
- Cycloalkane. Provided by: Wikipedia. Located at: http://en.wikipedia.org/wiki/Cycloalkane. License: Public Domain: No Known Copyright
This chapter is an adaptation of the chapter "Alphiatic Hydrocarbons" in Boundless Chemistry by LumenLearning and is licensed under a CC BY-SA 4.0 license.
molecule containing carbon and hydrogen that has only single bonds between carbon atoms
A compound consisting only of carbon and hydrogen atoms.
The thermal decomposition of a substance, especially that of crude petroleum, in order to produce petrol/gasoline.
A group of atoms joined by covalent bonds that are characterized by a free, unpaired electron that imparts their reactivity.
molecule consisting of only carbon and hydrogen atoms connected by single (σ) bonds
Molecules with the same number of atoms in different geometric arrangements.

