School of Medicine
68 Structural Motifs of Excitatory Synapses in the Mammalian Retina
Taylor Otterness; Crystal Sigulinsky; James R. Anderson; and Bryan W. Jones
Faculty Mentor: Crystal L. Sigulinksy (Ophthalmology, University of Utah)
Purpose
Connectivity within the nervous system is precise and disruptions lead to degraded performance and disease, yet the rules that govern connectivity largely remain unknown. Recent efforts reveal that distinct types of cone bipolar cells in the neural retina show preferences in the selection and frequency of presynaptic structure types used for signal transmission (Sigulinsky et al., 2020; Yu et al. 2023). However, it is not yet known how these differences are related to the quantity or type of postsynaptic partner. We used Retinal Connectome 1 (RC1) to analyze the synaptic output of rabbit CBb6 cells, a type of ON cone bipolar cell that forms excitatory synapses via diverse presynaptic structure types, to identify patterns in how these cells interact with their postsynaptic partners.
Methods
RC1 is a 0.25 mm diameter volume sampled from mid-peripheral retina of a 13-month-old female Dutch-Belted rabbit, serially sectioned at 70 nm, and imaged at ultrastructural resolution (2nm/px) using transmission electron microscopy (Anderson et al., 2011). Postsynaptic partners of CBb6 cell 6156’s presynaptic structures were annotated using the Viking Viewer for Connectomics. Statistical analyses were conducted in Microsoft Excel and investigated further with 3D rendering and graph visualization of connectivity.
Results
The factors tracked for comparison included presynaptic structure type, target number, and postsynaptic partner type. Cone bipolar cells use 3 types of excitatory chemical synaptic structures: single ribbons, multiribbons, and ribbonless synapses (Figure 1A-C). Single ribbons have 1 ribbon, multiribbons have >1 ribbon, and ribbonless structures lack a ribbon but have ≥2 synaptic vesicles tethered to the presynaptic membrane.
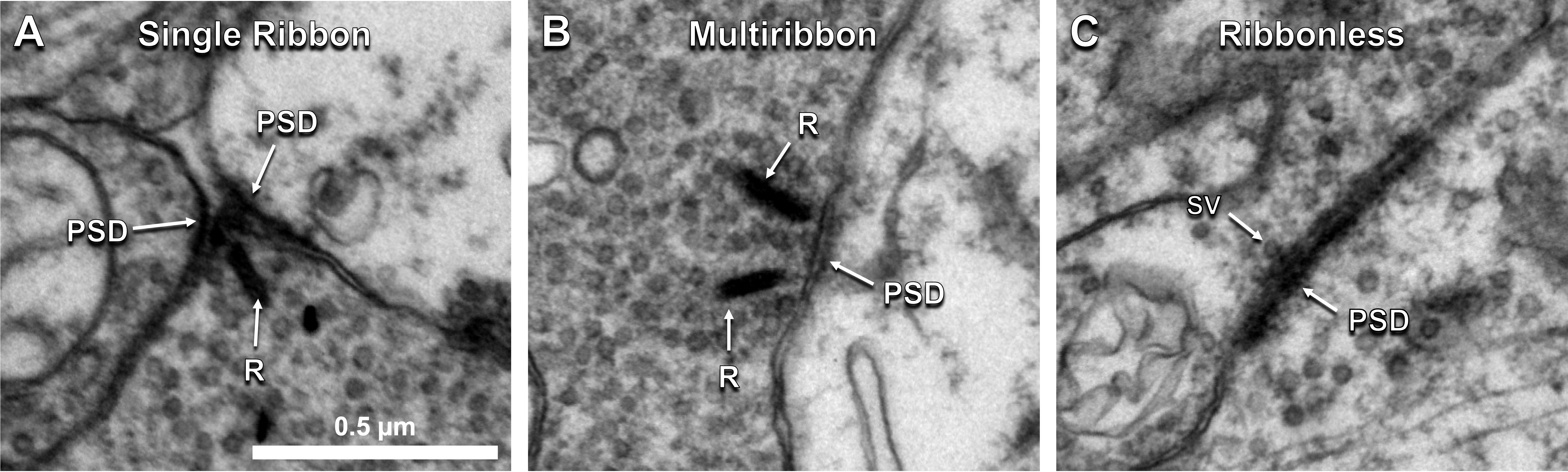
Figure 1. Cone bipolar cells form diverse presynaptic structure types. A) Single ribbon, B) multiribbon, and C) ribbonless presynaptic structure types observed at cone bipolar cell excitatory synapse sites in the rabbit retina. Abbr: R, ribbon; PSD, postsynaptic density; SV, synaptic vesicle.
The distribution of all excitatory presynaptic structures on CBb6 cell 6156 was modeled in 3D space and did not show a clear pattern in the distribution of presynaptic structure type based on depth or organization of the axonal arbor (Figure 2A-A’). Most presynaptic structures were located in the ON inner plexiform layer. The proportions of synapses of each presynaptic structure type with 1, 2, 3, or 4 postsynaptic partners were tracked and compared, which showed that multiribbon synapses of 6156 trended towards having a greater number of output partners (Figure 2B). Specifically, multiribbons overall had a greater proportion of 2 partner outputs (dyads) than 1 partner outputs (monads). Previous hypotheses proposed that presynaptic structure types may differ in the strength of neurotransmitter release (ribbonless < single ribbon < multiribbon). However, 3 partner and 4 partner outputs were only found opposing single ribbon presynaptic structure types (Figure 2C). These findings are inconsistent with scaling of output to the number of postsynaptic targets.
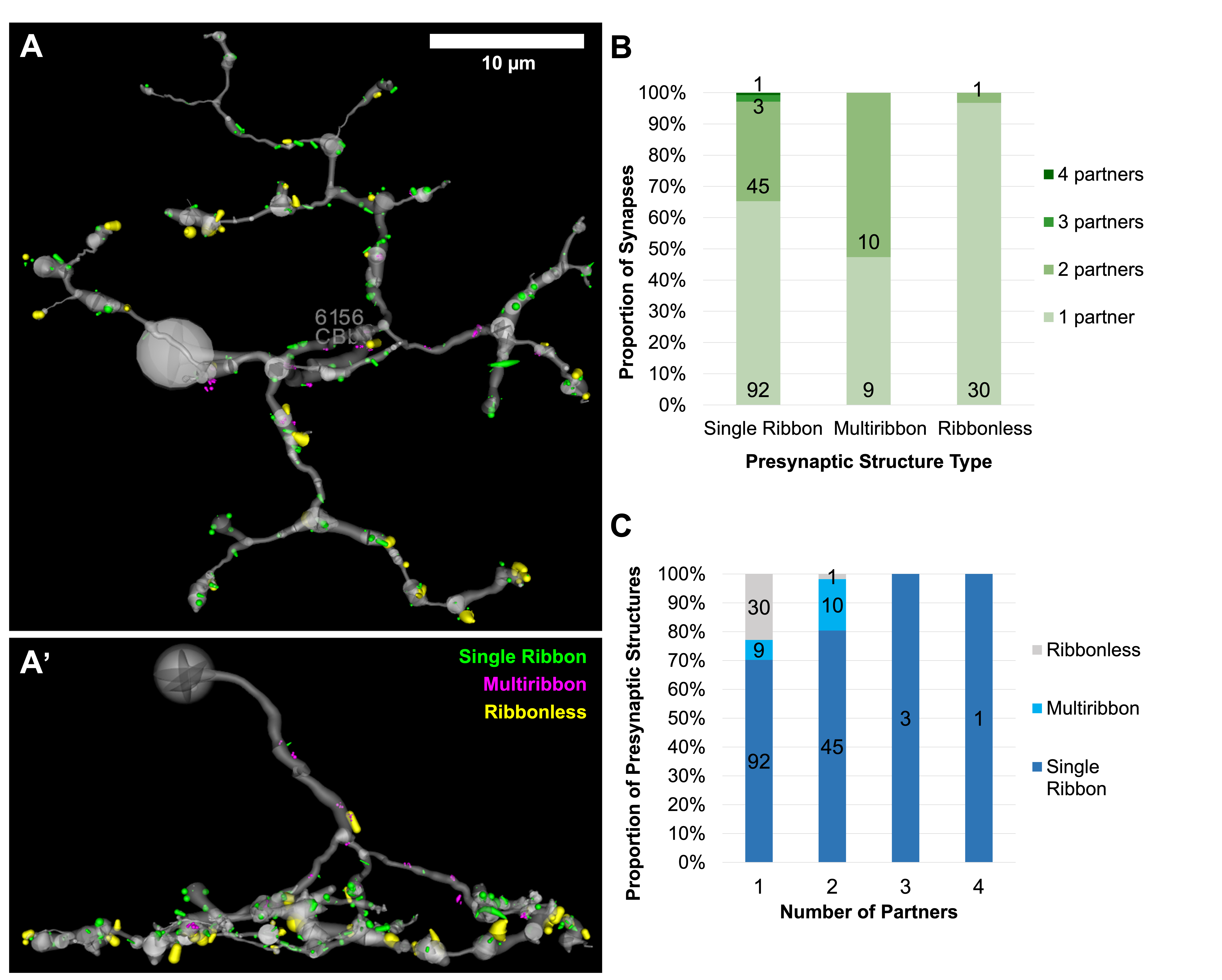
Figure 2. Distribution and relationships of presynaptic structure types of CBb6 6156. A) Top down (XY) view of the 3D rendered axonal arbor of CBb6 cell 6156 (gray) and presynaptic structures of its excitatory output synapses: single ribbon (green), multiribbon (magenta), and ribbonless (yellow). Structures scaled by a factor of 2 for visualization. A’) Side (XZ) view. B-C) Proportion of synapses of each presynaptic structure type with 1, 2, 3, or 4 postsynaptic partners.
Both amacrine cells (AC) and ganglion cells (GC) are postsynaptic partners of CBb6 cell 6156. However, single ribbon and ribbonless structures appear biased towards AC only targets at individual synapses, while multiribbon synapses appear biased towards having mixed AC and GC targets at individual synapses (Figure 3). In one case, cell 6156 served as the presynaptic partner synapsing with AC 5575 in five different locations. The fact that four of the five synapses in this partnership were multiribbons further supports the idea that postsynaptic partner type plays an important role in presynaptic structure selection with a high level of specificity.
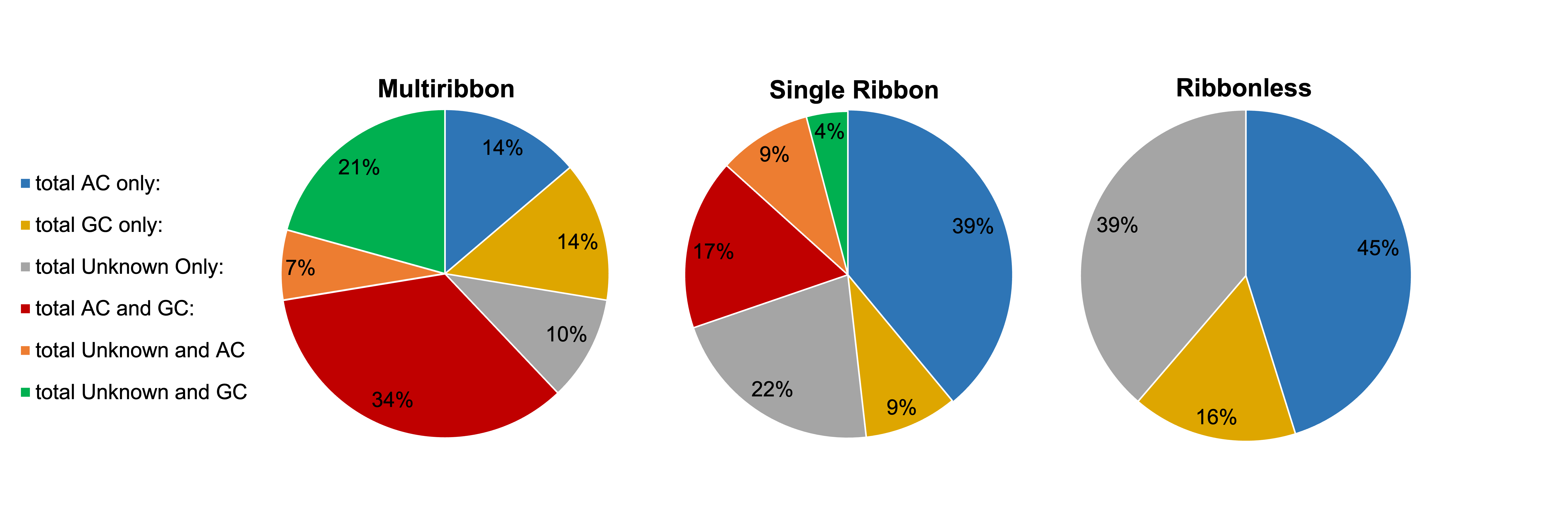
Figure 3: Partner classification proportions by presynaptic structure type. This figure does not account for quantity of output partners, therefore a presynaptic structure falling under the “AC only” output(s) category could have a single AC partner or multiple. Since ribbonless synapses did not form any dyads in this cell, they have fewer possible graphical categories.
Conclusion
It is hypothesized that presynaptic structure types may differ in the strength of neurotransmitter release (ribbonless < single ribbon < multiribbon), but the data collected for cell 6156 is inconsistent with such scaling of output to the number of postsynaptic targets. The data suggests that target type relationships may be more important than the number of targets in determining presynaptic structure type in CBb6 cells. This is consistent with recent work suggesting that cone bipolar cells in the mouse retina are also biased in their presynaptic structure type according to postsynaptic partner type (Yu et al., 2023). Future efforts will incorporate size differences of postsynaptic structures and presynaptic ribbon size, as well as compare across bipolar cell classes, in order to elucidate connectivity rules underlying excitatory synapses in retina.
Acknowledgements
This research was funded by the University of Utah Office of Undergraduate Research through the Undergraduate Research Opportunities Program, NIH R01 EY015128, NIH R01 EY028927 to B.W.J., NIH P30 EY014800 to Core, and NIH T32 EY024234 to C.L.S.; an NSF NN2 2014862 to B.W.J.; an unrestricted grant from Gabe Newell to B.W.J., and a Research to Prevent Blindness (New York) Unrestricted Grant to the Department of Ophthalmology & Visual Sciences, University of Utah. Funding for the JEOL JEM-1400 TEMs generously provided by the late Martha Ann Healy and the Bloomberg Family.
References
Anderson, J. R., Jones, B. W., Watt, C. B., Shaw, M. V., Yang, J. H., Demill, D., Lauritzen, J. S., Lin, Y., Rapp, K. D., Mastronarde, D., Koshevoy, P., Grimm, B., Tasdizen, T., Whitaker, R., & Marc, R. E. (2011). Exploring the retinal connectome. Molecular vision, 17, 355–379.
Sigulinsky, C., Anderson, J. R., Kerzner, E., Rapp, C., Pfeiffer, R. L., Rodman, T. M., Emrich, D., Rapp, K., Nelson, N. T., J. Scott Lauritzen, Meyer, M., Marc, R. E., & Jones, B. W. (2020). Network Architecture of Gap Junctional Coupling among Parallel Processing Channels in the Mammalian Retina. The Journal of Neuroscience, 40(23), 4483–4511.
Yu, W.-Q., Swanstrom, R., Sigulinsky, C., Ahlquist, R. M., Knecht, S., Jones, B. W., Berson, D. M., & Wong, R. (2023). Distinctive synaptic structural motifs link excitatory retinal interneurons to diverse postsynaptic partner types. Cell Reports, 42(1), 112006–112006.

