School of Medicine
49 Developing an Effective Drug-Screening Assay for Treating Cardiac Energetic Deficiencies
Ashley Cluff; Dipayan Chaudhuri; Sandra Lee; and Enrique Balderas
Faculty Mentor: Dipayan Chaudhuri (Internal Medicine, University of Utah)
The mitochondrial calcium uniporter (MCU) may help compensate for energetic deficiencies characteristic of some heart diseases, via a mechanism dependent on breaking an interaction between its N-terminal domain (NTD) and complex I of the electron transport chain [1]. Drugs that inhibit this interaction may offer new treatments for such heart diseases, but to identify such compounds we need a screening assay for MCU NTD interactions. The MCU NTDs also mediate dimerization of the MCU. To quantify the MCU NTD interactions, we used fluorescence resonance energy transfer (FRET), the process through which energy is transferred from a donor to an acceptor fluorescent protein, in this case mCerulean and mVenus respectively (figure 1). This process is highly distance dependent as the FRET efficiency is inversely proportional to the sixth power of the separation between the fluorescent proteins.
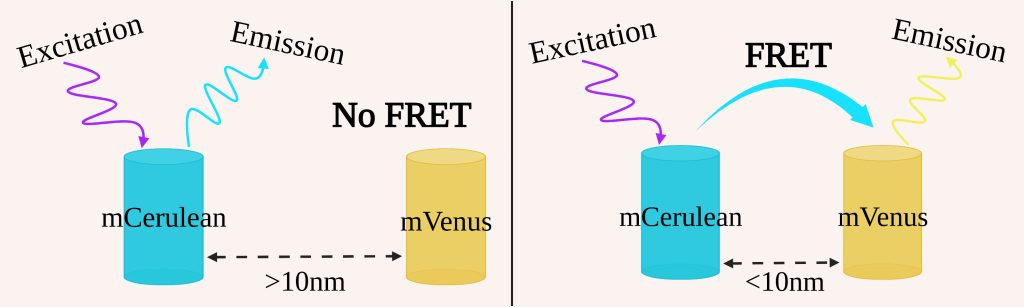 Figure 1: Process of FRET
Figure 1: Process of FRET
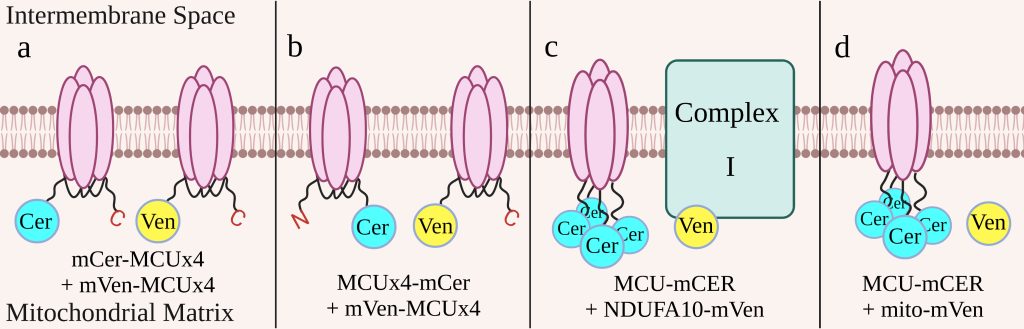 Figure 2: Cell Constructs
Figure 2: Cell Constructs
We constructed four cell lines to determine which construct would be the most effective indicator of drug effectiveness (figure 2). The first two cell lines have the MCU subunits linked to one another. One cell line has mCerulean and mVenus, both on the N-terminus of the linked MCU (figure 2a), while the other has mCerulean on the C-terminus and mVenus on the N-terminus (figure 2b). These cell lines will take advantage of the dimerization of MCU to produce FRET. The last two cell lines have each of the four MCU subunits tagged with mCerulean. One cell line also has NDUFA10, a subunit of complex I, tagged with mVenus (figure 2c) and uses the interaction between complex I and the MCU to produce FRET. The other has mVenus free in the mitochondrial matrix (figure 2d) and is used as a control to determine background levels of FRET. Each cell construct has a bidirectional promoter that ensures both elements of the construct are expressed at similar levels. This promoter is activated by doxycycline, so we are able to control when the constructs are expressed in the cells in order to minimize the risk that the constructs may harm the cell. To prevent immature fluorescent proteins, which would lead to an underestimate of the FRET efficiency, we treated the cell lines with cycloheximide, a protein synthesis inhibitor, before measuring fluorescence with flow cytometry.
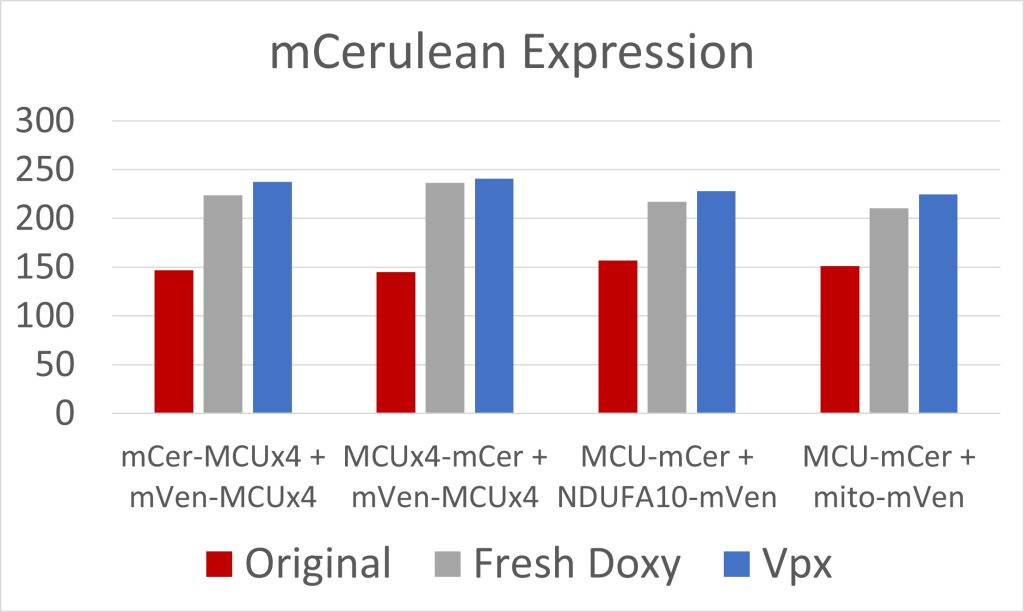 Figure 3: mCerulean Expression
Figure 3: mCerulean Expression
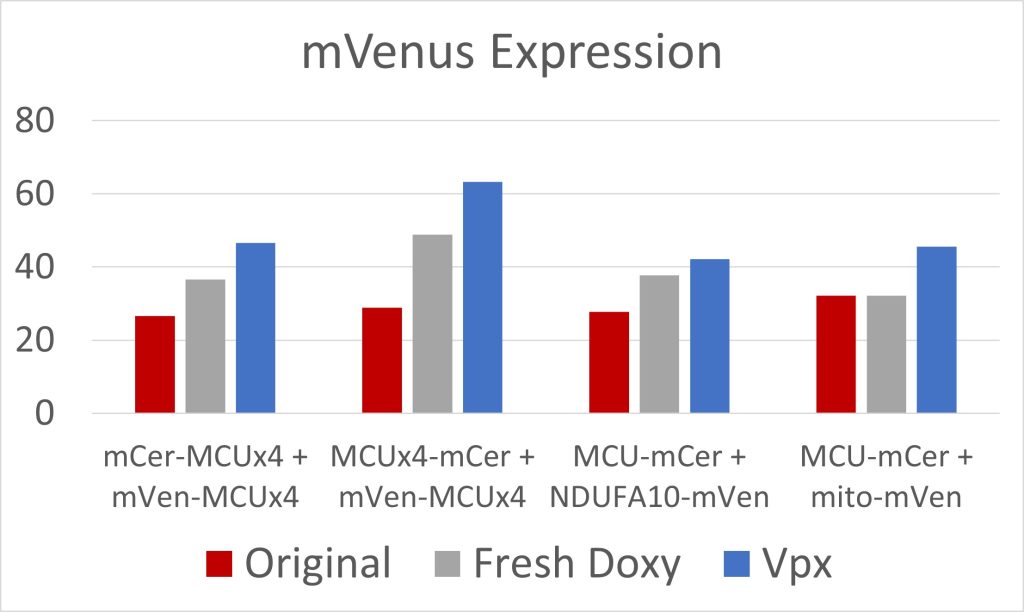
Figure 4: mVenus Expression
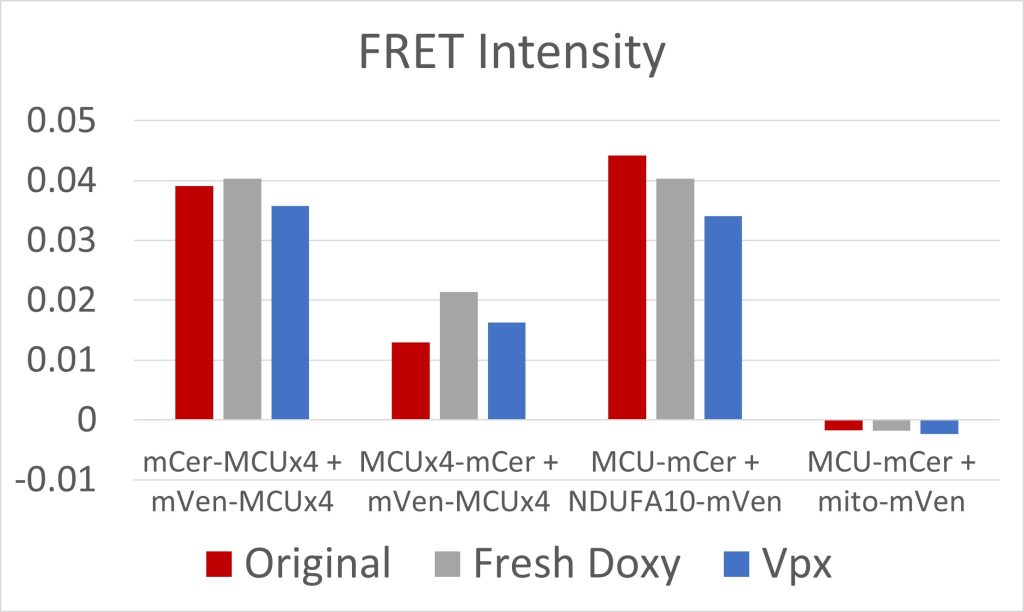 Figure 5: FRET Intensity
Figure 5: FRET Intensity
Due to low initial MCU expression in the cells, we used fresh doxycycline and transfected the cells with Vpx, a viral protein that has allowed for the transcription of long coding mRNA in similar cells [2]. Both were effective at increasing MCU expression (figures 3 and 4). The cell constructs with mCerulean and mVenus on the N-terminals, and mCerulean-tagged MCU with mVenus-tagged NDUFA10, exhibited the most FRET because of the closer proximity of the fluorescent proteins due to the NTD interactions involved in both dimerization of the MCU and MCU interactions with complex I (figure 5). These constructs will be used to quantify the efficacy of drugs in breaking the interaction between complex I and the MCU. Additionally, we are creating constructs where mCerulean and mVenus are linked to one another through varying numbers of amino acids. We would expect the constructs with the shortest linkers to have the most FRET. These standards can be used to calculate the FRET efficiency, as opposed to the FRET intensity found simply by measuring fluorescence. FRET efficiency is the proportion of donor fluorescent proteins which have transferred their excitation energy to the acceptor fluorescent proteins. The FRET efficiency value is important for comparing FRET across different instruments and throughout time due to instrument wear.
References
[1] Balderas, , Eberhardt, D.R., Lee, S. et al. Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment. Nat Commun 13, 2769 (2022). https://doi.org/10.1038/s41467-022-30236-4
[2] Yurkovetskiy, , Guney, M.H., Kim, K. et al. Primate immunodeficiency virus proteins Vpx and Vpr counteract transcriptional repression of proviruses by the HUSH complex. Nat Microbiol 3, 1354–1361 (2018). https://doi.org/10.1038/s41564-018-0256-x

