College of Health
29 Effects of Increasing Sleep Duration on C-reactive Protein, Insulin Sensitivity, and Blood Pressure in Adults with Habitual Short Sleep
Alisha Chong
Faculty Mentor: Christopher Depner (Health, University of Utah)
Abstract
Short sleep, cardiovascular disease, and type 2 diabetes are all highly prevalent health issues in modern society. Previous findings demonstrate that obtaining short sleep is linked to an increased risk of cardiometabolic diseases such as type 2 diabetes and heart disease. However, the mechanisms underlying how short sleep duration affects these health issues are not fully understood. C-reactive protein (CRP) is an inflammatory marker which has been linked to a greater risk of cardiovascular disease, but prior research has found conflicting information on whether short sleep significantly affects CRP levels. Thus, the current project aims to investigate the impact of short sleep on CRP levels as well as on insulin sensitivity and blood pressure to better understand the potential mechanisms by which short sleep duration is linked to adverse cardiovascular risk.
The project utilized data from the parent study, “Biomarkers and Altered Metabolic Pathways during Sleep Loss.” The study recruited healthy individuals aged 18-35 who chronically received short sleep (<6.5h per night). After baseline assessments, participants completed a 4-week intervention aiming to increase their time in bed by 2 hours per night. Sleep duration and physical activity data were collected throughout the study. Insulin sensitivity, CRP, blood pressure, and fasting glucose and insulin levels were compared at baseline and sleep extension.
Of the 20 participants included in the current analysis, average sleep duration was 340 ± 83 (± SD) minutes per night at baseline and significantly increased (p < 0.001) by 61 ± 11 (± SE) minutes during sleep extension. Insulin sensitivity after sleep extension was lower than baseline. Specifically, in a linear mixed-effects regression model adjusting for sex, body weight, and moderate-to-vigorous physical activity (MVPA), the Matsuda index significantly decreased (P < 0.05) by 1.2 ± 0.5 (SE) and HOMA-IR significantly increased (P < 0.05) by 0.4 ± 0.2 (SE) from baseline to extension. No significant differences between segments were observed for CRP, blood pressure, or fasting glucose and insulin levels. Systolic blood pressure was higher for men than for women at baseline, suggesting that among individuals habitually receiving short sleep, men may be at higher risk of hypertension. Our current findings highlight that otherwise healthy young adults with low overall risk of cardiometabolic disease do not show physiological benefit from a 4-week sleep extension intervention. The current intervention was feasible and increased sleep duration by ~1 hour per night. Longer term interventions in higher-risk populations such as older adults with obesity are needed to more comprehensively quantify the impact of sleep extension on risk of cardiometabolic disease.
Introduction
A vital aspect of maintaining overall good health is obtaining adequate sleep. Cardiovascular and metabolic health are no exception to this need. As such, the American Academy of Sleep Medicine and the Sleep Research Society recommend adults obtain seven or more hours of sleep each night for optimal health.[1] However, a large portion of adults are not meeting this recommendation. A report from the Centers for Disease Control and Prevention (CDC)’s Preventing Chronic Disease journal shows in 2020, 33.2% of adults in the US reported receiving fewer than the recommended seven hours of sleep per night.[2] Prior studies demonstrate that obtaining short sleep duration increases risk of several diseases and health conditions, many of which are interconnected. For instance, poor quality sleep and short sleep are associated with an increased risk of type 2 diabetes,[3] and evidence shows short sleep duration leads to decreased insulin sensitivity.[4] Reduced insulin sensitivity may develop into prediabetes and eventually insulin resistance and type 2 diabetes, which increases risk of early mortality.
Few studies have explored whether increasing sleep duration is a viable and practical way to improve insulin sensitivity in people who obtain habitual short sleep durations. Broussard et al. conducted a study testing the effects of acute sleep deprivation followed by sleep recovery on insulin sensitivity in healthy young men, assigning them to complete, in random order, four nights of normal sleep and four nights with restricted sleep followed by two nights of recovery sleep under laboratory conditions.[5] Under this protocol, participants displayed impaired insulin sensitivity after sleep restriction and improvement in insulin sensitivity after recovery sleep,[5] suggesting that recovery sleep or sleep extension is a feasible method of improving insulin sensitivity after acute sleep deprivation. These intriguing laboratory-based findings warrant further investigation in people with habitual short sleep duration in the real-world setting. In adults with chronic sleep deprivation, another study by Leproult et al. found that six weeks of at-home sleep extension during weekdays improved a fasting marker of insulin sensitivity.[6] Their results indicate that interventions to increase sleep duration in short sleepers may have potential to increase insulin sensitivity and reduce metabolic disease risk. Such interventions are a promising avenue for further investigation.
Short sleep duration is also linked to increased risk of cardiovascular diseases such as coronary heart disease and stroke.[7] However, the precise mechanisms by which this occurs are not fully understood. As mentioned previously, short sleep duration increases the risk of type 2 diabetes, which also increases the risk of heart disease as high blood sugar causes damage to blood vessels.[8] Furthermore, sleep deprivation is associated with high blood pressure.[9] High blood pressure is a common comorbidity of type 2 diabetes, and both may lead to cardiovascular disease through means such as development of atherosclerosis and inflammation.
The processes linking atherosclerosis and inflammation to cardiovascular disease involve immune cell leukocytes. Endothelial cells of the arteries experience inflammation when a plaque begins to form.[10] Though leukocytes do not typically bind to this endothelium, the inflamed endothelium expresses adhesion molecules which do bind to classes of leukocytes associated with arterial plaques.[10] The activity of these leukocytes has the potential to weaken the plaque’s fibrous cap attachment, increasing the risk of plaque rupture.[10] This rupture can go on to cause heart diseases like heart attack or stroke. Thus, inflammation may both mediate and indicate risk of cardiovascular disease, especially heart attack and stroke.
As such, one potential measure of cardiovascular disease risk is the body’s level of inflammatory markers such as high-sensitivity C-reactive protein (hs-CRP).[11] CRP has been linked to higher stroke and heart attack risk.11 Evidence supports CRP playing a role in upregulation of adhesion molecule expression,[11] which may help mediate the aforementioned process involving inflammation, immune cell activation, atherosclerosis and thrombosis, and cardiovascular disease. Because short sleep duration might increase heart disease risk through increasing inflammation,[9] it is possible CRP levels may serve as an indicator of short sleep duration or as a clue towards a specific molecular mechanism through which short sleep increases heart disease risk.
However, there have been conflicting findings on how sleep duration impacts CRP levels. One study by Chiang in Taiwanese adults found that after adjusting for potential confounding factors, individuals with a self-reported sleep time of less than 5.5 hours per day, including naps, had over twice as high a risk of increased CRP levels than those sleeping longer.[12] Another study by Grandner et al. investigating self-reported extreme nighttime sleep durations and CRP levels across different racial and ethnic groups found that CRP levels tend to be elevated in people with long sleep duration but not significantly in people with short sleep duration.[13] These results varied by group, and contradictory to Chiang’s study, Grandner et al.’s results found that in Asian participants, short sleep duration appears to protect against elevated CRP levels. The Asian group in Grandner et al.’s study, however, included a larger variation in ethnicities (labelled Asian/Other). For non-Hispanic White participants, CRP levels were elevated in individuals with long sleep duration. Hispanic/Latino participants did not display significantly elevated CRP levels at any sleep duration after adjustments, and for Black/African American participants, CRP levels were elevated in individuals sleeping fewer than five hours and in individuals sleeping eight hours.[13] A different study in nurses of various racial and ethnic groups from two Texas hospitals found no differences in CRP levels by actigraphy nor diary-reported total sleep time in White, Black, and Asian participants but did find elevated CRP with higher diary-reported total sleep time in Hispanic participants.[14] Other studies have found increases in CRP after acute total and partial sleep restriction,[15,16] and one found an association in middle-aged to older adults between daytime napping and elevated CRP and between longer time spent in bed and elevated CRP but found no association with sleep duration.[17]
These conflicting results regarding the impact of sleep duration on CRP, the incomplete understanding of the molecular mechanisms linking short sleep to increased heart disease risk and diabetes risk, and the need for further investigation regarding increasing sleep duration to improve insulin sensitivity and metabolic disease risk in people with habitually short sleep all represent gaps in knowledge. These gaps limit the development of sleep-based countermeasures that could help reduce the risk of cardiometabolic disease in people with short sleep duration. This poses an issue given the current high prevalence of short sleep in adults as well as the high incidence of damaging diseases like heart disease and type 2 diabetes in the US. More research will help advance our understanding of the associations between sleep duration and cardiometabolic disease risk. Although the current project does not study enough participants from different racial and ethnic groups to conduct a stratified analysis, it does aim to gather more data on the effects of sleep duration on CRP levels as well as on factors impacting heart disease risk such as insulin sensitivity and blood pressure.
The current project analyzed healthy individuals aged 18-35 chronically receiving short sleep. Various measures such as fasting glucose and insulin levels, CRP, insulin sensitivity, and vital signs of participants were compared at baseline and after a four-week intervention aiming to increase time in bed by 2 hours per night to determine whether significant differences existed before and after the sleep intervention. The project’s hypothesis was that increasing sleep duration of people chronically receiving <6.5h of sleep per night would lower plasma CRP levels, decrease blood pressure, and increase insulin sensitivity.
Methods
Data for this project were collected in the parent study “Biomarkers and Altered Metabolic Pathways during Sleep Loss” conducted by the Sleep and Circadian Physiology Lab run by Dr. Christopher Depner in the University of Utah Department of Health and Kinesiology. All study procedures were approved by the University of Utah Institutional Review Board, and all participants
provided written informed consent prior to initiating any study procedures. The study recruited healthy individuals aged 18-35 who reported habitually obtaining fewer than 6.5 hours of sleep per night. Participants had a Body Mass Index (BMI) within the 18.5-24.9 range and reported fewer than 150 minutes of moderate-to-vigorous physical activity (MVPA) per week. Participants also lived at or above the altitude of Salt Lake City for at least three months prior to beginning the study and stayed within the same time zone for at least three weeks prior to beginning the study. Individuals who reported night shift work within a year prior to beginning the study and individuals diagnosed with or displaying symptoms of clinically significant sleep disorders were excluded. Initial screening of applicants to the study was completed using an online REDCap survey with questions on applicants’ medical history and conditions, habitual sleep and waketimes, and typical sleep onset latency. Prior to visit 1, potential participants also received additional REDCap surveys to further evaluate sleep history.
Data collection for each participant in the sleep study occurred over the course of nine visits. Visits 3 through 6 occurred during the baseline phase, where participants would maintain their regular sleep habits and schedule for two weeks at home. Visits 7 through 9 occurred during the sleep extension intervention phase, where participants would aim to increase their time in bed by 2 hours per night for four weeks. Participants were asked to refrain from napping or remaining awake for an entire night at any point during baseline and sleep extension. At visit 1, lab staff explained study procedures and obtained informed consent and additional medical and psychological health history as well as current drug and medication use. Additional medical screening was conducted at visit 2, and an overnight polysomnography sleep assessment was performed at visit 4 at the University of Utah Health Sleep-Wake Center. In the case that participants were unable to be evaluated at the Sleep-Wake Center, they were given an Apnea Link device to conduct an at-home sleep assessment.
Sleep duration was measured throughout the study by wrist actigraphy using the Actiwatch Spectrum by Philips Respironics. Sleep and waketimes were recorded throughout the study in paper and electronic sleep diaries. An activPALTM monitor by PAL Technologies was worn on the thigh to determine physical activity throughout the two-week baseline period and during the last two weeks of sleep extension. The Actiwatch, sleep diary, and activPALTM device were given at visit 3, and participants would begin baseline monitoring that night. Participants received a Dreem headband (by Beacon Biosignals, Inc.) at visit 5 to track their brain activity at home while sleeping for the last week of baseline.
Visit 6 was an overnight visit held at the University of Utah Hospital to conduct physiological baseline measurements. Participants arrived approximately three hours prior to their habitual bedtime as determined by their electronic sleep log entries. Their sleep was again assessed by polysomnography. Scheduled waketime was also determined using electronic sleep log entries. Vital signs and body measurements, including systolic and diastolic blood pressure and body weight, were measured during this visit. CRP levels were measured from fasting plasma samples. Oral Glucose Tolerance Testing (OGTT) administered one hour after waketime provided the fasting, 30-, 60-, 90-, and 120-minute plasma glucose (PG) and insulin levels for use in calculating measures of insulin sensitivity.
After visit 6, participants were instructed to increase their time in bed by 2 hours per night for the next four weeks. To facilitate the sleep extension, the study team worked with participants to set individualized sleep and waketime targets. To help participants increase their sleep duration, staff also provided handouts containing advice from the National Sleep Foundation. Examples of advice included keeping the bedroom temperature cool, maintaining consistent bed and waketimes, avoiding caffeine intake during the afternoon, and taking a hot shower as well as avoiding food and alcohol, exercise, and blue light exposure in the one to two hours before bed. Participants continued to use the Actiwatch and sleep diaries to track sleep duration and sleep and waketimes. At visit 7, the
activPALTM device was once again provided to them to track their physical activity for the last two weeks of sleep extension. Visit 7 also served as a check-in visit to ensure the participants were maintaining their target sleep and waketimes and to provide any guidance necessary to maintaining or reaching these goals. The Dreem headband was given again at visit 8 to track brain activity while sleeping at home for the last week of sleep extension. Finally, visit 9 was conducted under the same procedures as visit 6, with scheduled arrival, bed, and waketimes determined by the extension period entries of the electronic sleep log.
For the current project, Dreem data were used to calculate total sleep times. To determine participant insulin sensitivity, OGTT glucose and insulin data from visits 6 for baseline and 9 for extension were used to calculate Matsuda index and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Linear mixed-effects regression models were created in R to test for statistically significant differences between baseline and extension measurements of Matsuda index, HOMA-IR, CRP (mg/L), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), fasting glucose (mg/dl), fasting insulin (microU/ml), and total sleep time (min). The primary model (model 1) adjusted for sex and body weight only. A second model (model 2) adjusted for sex, body weight, and moderate-to-vigorous physical activity. Statistical significance was set at an a of 0.05.
Results
After exclusions due to missing or outlier data, baseline and extension data from 20 participants (totaling to 36 observations for glucose, insulin, and insulin sensitivity indices and 38 observations for blood pressure and CRP in R data frame) were included in our current analysis. Ten participants were female, and ten were male. Total sleep time per night was 340 ± 83 (± standard deviation) minutes on average at baseline. After adjusting for sex in a linear mixed-effects regression model, the total sleep time significantly increased by 61 ± 11 minutes (± standard error, p < 0.001) during sleep extension. The average nightly total sleep time during sleep extension was 398 ± 84 (± SD) minutes, a 17% increase from baseline (Figure 1).
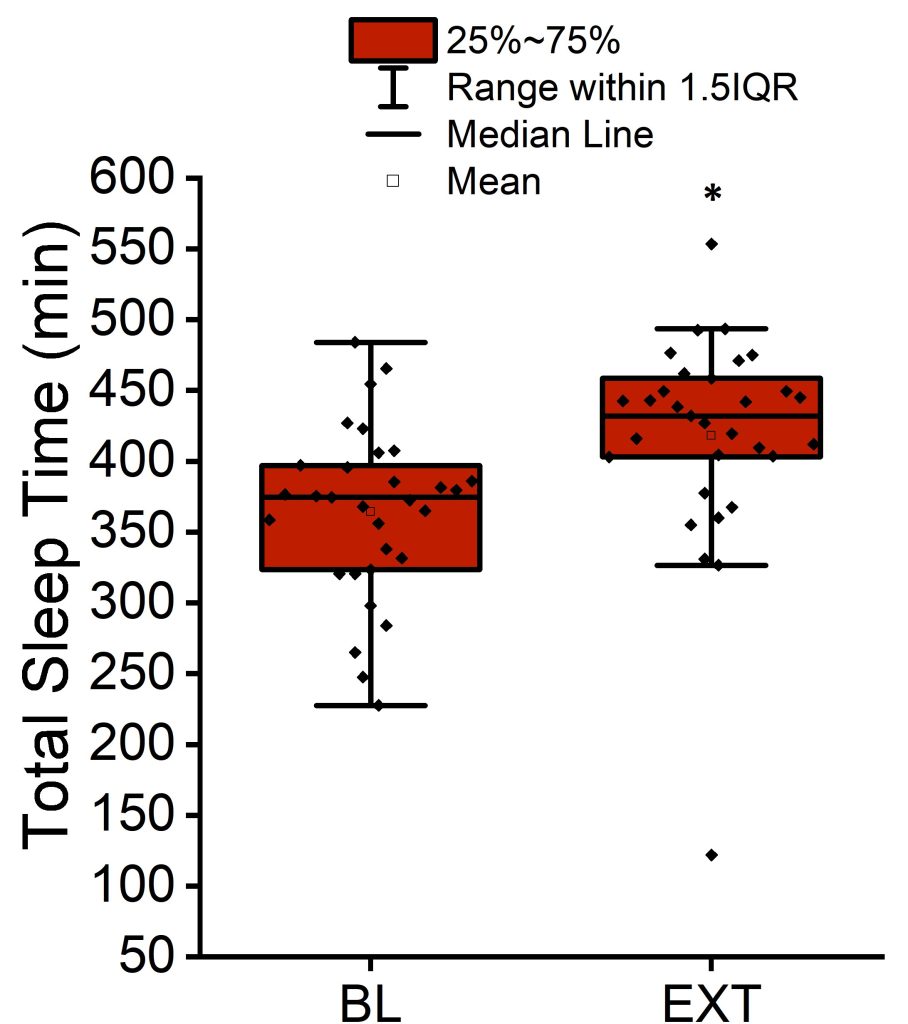 Figure 1. Mean, median, interquartile range, and 1.5 interquartile range of the total sleep time per night at baseline and after sleep extension. *p < 0.001 versus baseline (BL).
Figure 1. Mean, median, interquartile range, and 1.5 interquartile range of the total sleep time per night at baseline and after sleep extension. *p < 0.001 versus baseline (BL).
The Matsuda index average was 6.7 ± 2.7 (± SD) at baseline and decreased by 22% by the end of extension (p < 0.01; Figure 2 and Table 1). The HOMA-IR average was 1.3 ± 0.5 (± SD) at baseline and increased by 26% by the end of extension (p < 0.05; Figure 3 and Table 1).
Table 1. Average values and number of observations in baseline and extension insulin sensitivity indices, blood pressure, CRP, and fasting plasma glucose and insulin. *p < 0.01 versus baseline (BL). **p < 0.05 versus baseline (BL).
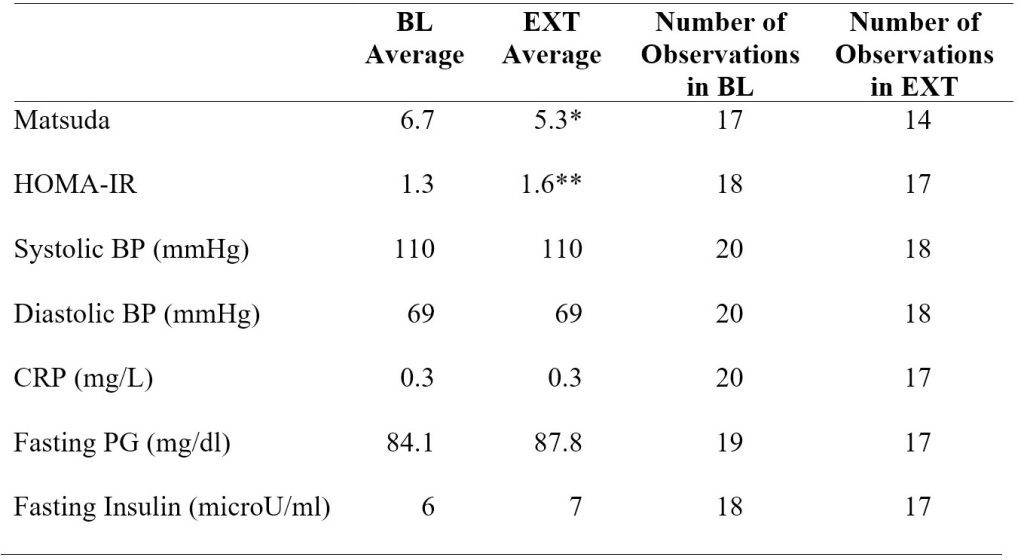
Figure 2. Mean, median, interquartile range, and 1.5 interquartile range of the Matsuda index measuring insulin sensitivity at the end of baseline period and after completion of sleep extension. *p < 0.01 versus baseline (BL).
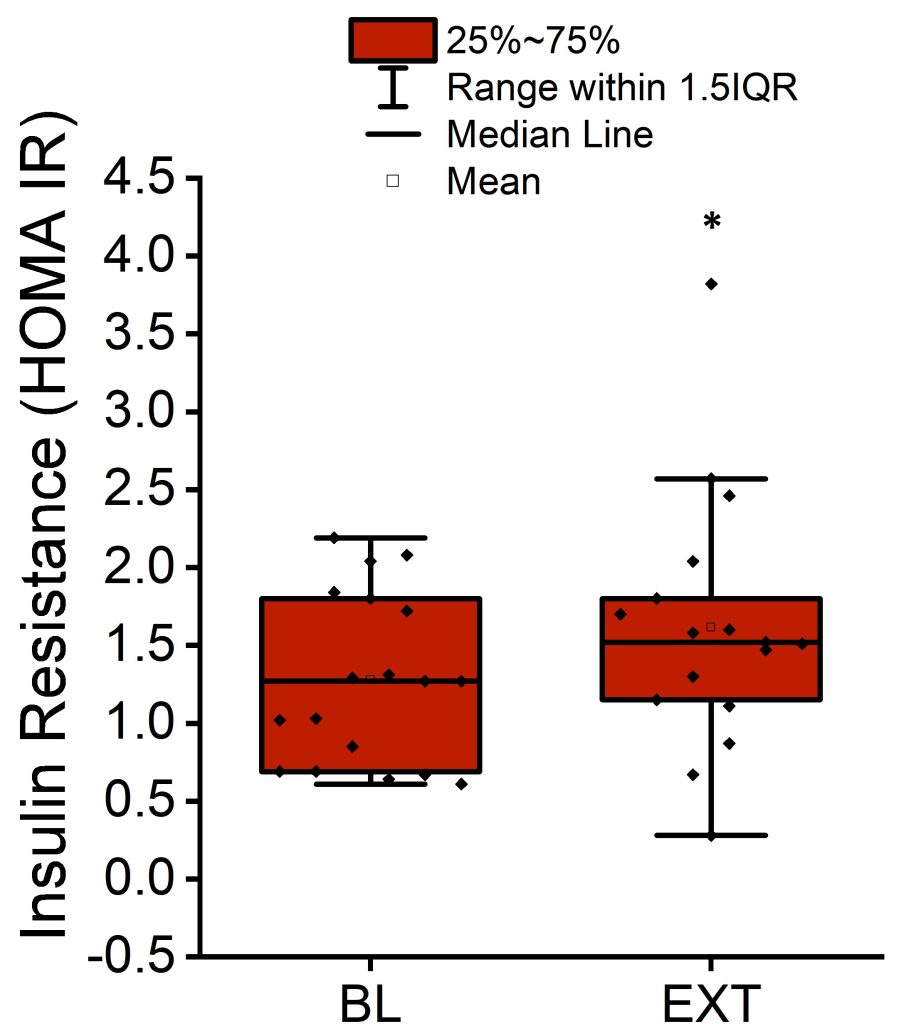
Figure 3. Mean, median, interquartile range, and 1.5 interquartile range of HOMA-IR measuring insulin resistance at the end of baseline period and after completion of sleep extension. *p < 0.05 versus baseline (BL).
In model 1 adjusting for sex and body weight, the Matsuda index significantly decreased (P < 0.01) by 1.1 ± 0.4 (SE) from baseline to extension. HOMA-IR significantly increased (P < 0.05) by 0.4 ± 0.1 (SE) from baseline to extension (Table 2).
Table 2. Model 1 linear mixed-effects regression analysis results for insulin sensitivity indices, blood pressure, CRP, and fasting plasma glucose and insulin. Model 1 adjusted for sex and body weight. *p < 0.01 versus baseline (BL). **p < 0.05 versus baseline (BL).
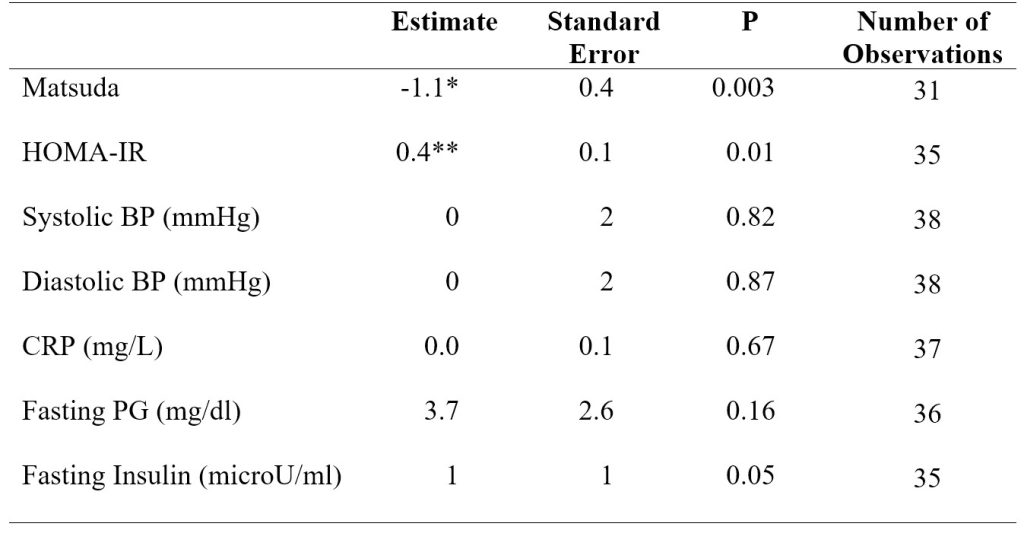 In model 2 adjusting for sex, body weight, and MVPA, the Matsuda index again significantly decreased (P < 0.05) by 1.2 ± 0.5 (SE) from baseline to extension. HOMA-IR significantly increased (P < 0.05) by 0.4 ± 0.2 (SE) from baseline to extension (Table 3). Adding MVPA as a covariate in model 2 decreased the number of observations in the regression analysis due to a large number of MVPA data being missing. Running the analysis for systolic blood pressure and for fasting plasma glucose in model 2 resulted in a singular fit error due to the shortage of data.
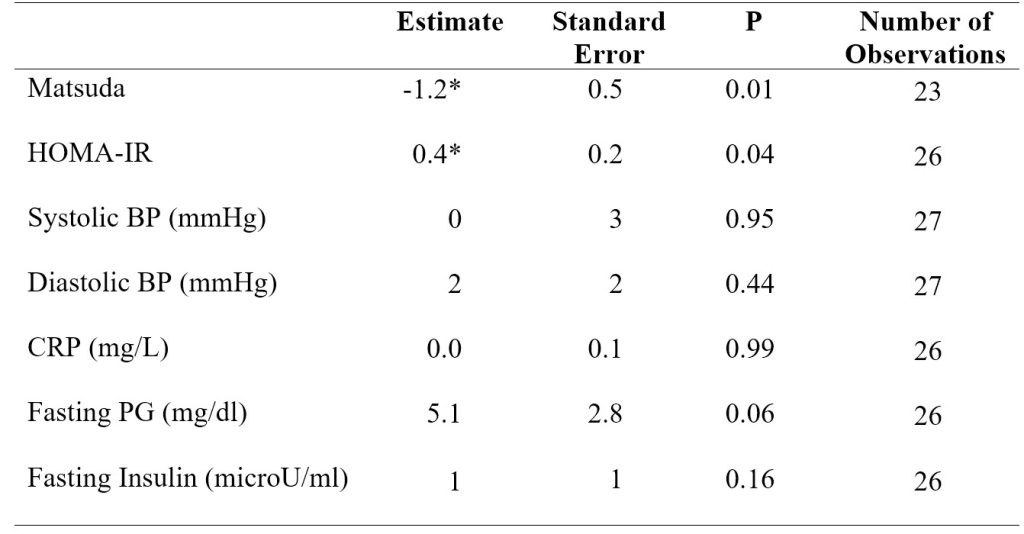
In model 2 adjusting for sex, body weight, and MVPA, the Matsuda index again significantly decreased (P < 0.05) by 1.2 ± 0.5 (SE) from baseline to extension. HOMA-IR significantly increased (P < 0.05) by 0.4 ± 0.2 (SE) from baseline to extension (Table 3). Adding MVPA as a covariate in model 2 decreased the number of observations in the regression analysis due to a large number of MVPA data being missing. Running the analysis for systolic blood pressure and for fasting plasma glucose in model 2 resulted in a singular fit error due to the shortage of data.
Table 3. Model 2 linear mixed-effects regression analysis results for insulin sensitivity indices, blood pressure, CRP, and fasting plasma glucose and insulin. Model 2 adjusted for sex, body weight, and MVPA. *p < 0.05 versus baseline (BL).
There were no significant differences between baseline and extension measurements of systolic blood pressure, diastolic blood pressure, CRP, fasting plasma glucose, or fasting plasma insulin. An exploratory linear regression model analyzing systolic blood pressure at baseline by sex did reveal a significant difference between systolic blood pressure in male and female participants. Systolic blood pressure was higher (P < 0.05) by 9 mmHg ± 4 mmHg (SE) in male versus female participants at baseline (Figure 4).
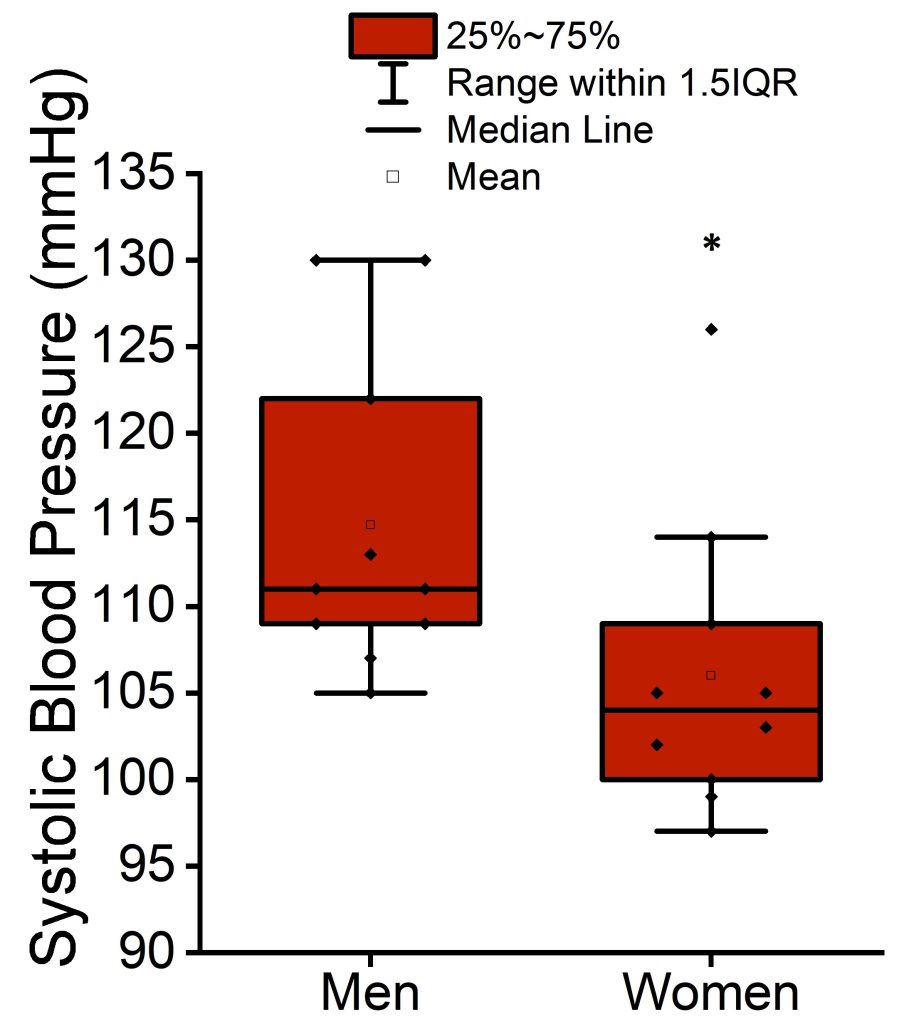 Figure 4. Mean, median, interquartile range, and 1.5 interquartile range of systolic blood pressure at baseline in men and women participants. *p < 0.05 versus women.
Figure 4. Mean, median, interquartile range, and 1.5 interquartile range of systolic blood pressure at baseline in men and women participants. *p < 0.05 versus women.
Discussion
In the current study, our findings show extending or increasing sleep duration in individuals who habitually obtain short sleep reduced insulin sensitivity. These findings were consistent for both the Matsuda index and HOMA-IR and in statistical models with or without MVPA included as a covariate, contrary to our hypothesis. This outcome is also inconsistent with findings from Leproult et al.’s study, which similarly investigated the effects of a sleep extension intervention in adults chronically receiving short sleep.[6] Both studies resulted in an approximately one-hour increase in sleep duration, though Leproult et al.’s study involved a six-week intervention compared to the current project’s four-week intervention. This longer duration may have contributed to the mixed findings between studies, and additional research with longer term interventions may be helpful to explain the observed differences.
One possible explanation for the decrease in insulin sensitivity in our data may lie in a potential adverse circadian shift driven by adapting to the sleep extension intervention. The current project did not control whether participants slept earlier, woke later, or both to increase their sleep duration. Additionally, though target bed and waketimes were provided, the participants did not strictly adhere to these times every day. There may be a possibility that participants’ circadian clocks were shifted so that melatonin offset was delayed to occur later after waking, causing melatonin levels to remain high at the time of insulin sensitivity testing.
Prior evidence demonstrates that high melatonin concentrations can decrease insulin sensitivity and glucose tolerance. One study found that providing 1 mg of exogenous melatonin to postmenopausal women in the morning after fasting resulted in reduced insulin sensitivity and glucose tolerance.[18] Moreover, another study examined a 5 mg dose of melatonin in young women and found that glucose tolerance was lower in the melatonin group compared to the placebo group.[19] Given this potential for altered melatonin to influence insulin sensitivity in our study, we are planning to analyze melatonin concentrations during the insulin sensitivity testing portion of the protocol. Future analysis of these melatonin samples would further the investigation into the possibility that an adverse circadian shift and delayed melatonin offset could contribute to the decrease in insulin sensitivity after sleep extension. If so, such findings would suggest that interventions targeting the timing and regularity of sleep may be more effective than interventions focused explicitly on increasing sleep duration.
Furthermore, if sleep extension did alter the timing of participants’ sleep schedules, the resulting sleep irregularity could explain the decrease in insulin sensitivity. Besides short sleep, irregular sleep is also highly prevalent in modern populations.[20] One study investigating sleep irregularity’s impact on metabolic syndrome using cross-sectional and prospective analyses on the participants in the Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated that variation above a certain level in both sleep duration and sleep onset timing was associated with a higher risk of metabolic syndrome.[20] Though this study did not explicitly investigate insulin sensitivity, metabolic syndrome is a condition closely linked to insulin resistance.[21] Additionally, a different analysis on women in the Study of Women’s Health Across the Nation (SWAN) Sleep Study observed increased insulin resistance with higher bedtime variability and with greater delay from habitual bedtime.[22]
Leproult et al.’s study specifically recruited participants who had differing sleep durations between weekdays and weekends, and their baseline measurements indicated the participants slept over an hour longer during weekends compared to weekdays.[6] As participants increased their sleep duration during the week but did not significantly change sleep duration on weekends,[6] this gap between weekday and weekend sleep duration would shrink during the sleep extension period, which could reflect a decrease in sleep duration variability. However, this would not necessarily mean reduced irregularity in sleep onset timing. The current project did not require nor investigate differences between weekday and weekend sleep schedules. Some participants reported more consistent daily sleep schedules in their sleep diaries while others had varying bed and waketimes throughout the week. Further analysis of sleep and waketimes to compare sleep regularity at baseline and during extension could help verify whether a link between sleep irregularity and decreased insulin sensitivity exists in the outcomes of the current project. Regardless, future study into sleep irregularity’s effect on insulin sensitivity and cardiometabolic risk factors would be a beneficial direction to further elucidate the link between poor sleep and increased risk of type 2 diabetes and heart disease.
The current project did not find any significant differences between baseline and extension measurements of CRP, systolic blood pressure, diastolic blood pressure, fasting glucose, or fasting insulin. The participants were young, healthy adults with low risk of cardiovascular disease, so their levels of these measures were likely normal to begin with and were minimally impacted by an increase
in sleep duration. However, systolic blood pressure was higher in men than in women at baseline. Thus, among short sleepers, men may be more susceptible to hypertension than women. This finding is consistent with existing knowledge on sex differences in blood pressure, which generally state that men have higher blood pressure, have higher prevalence of hypertension (except after the age that women reach menopause), and are at risk of cardiovascular disease at an earlier age.[23]
Expanding on this project to continue searching for clues behind the underlying mechanisms between short sleep duration and cardiometabolic disease risk would help advance development of precision medicine for these issues, such as by determining which individuals at which ages would benefit most from sleep extension interventions. As CRP, blood pressure, and fasting glucose and insulin were not significantly impacted by sleep extension in young adults with no observed health issues, future studies would focus on a population with higher cardiovascular and metabolic disease risk. These could include a cohort of older and overweight adults habitually receiving short sleep to determine if these individuals have elevated CRP and whether sleep extension would be an effective intervention to lowering CRP and chronic disease risk.
In regard to the current project, the results suggest men who receive short sleep may need to be more vigilant regarding hypertension risk than women. However, for the age range of young adults, sleep extension did not appear to be beneficial. As such, although the sleep intervention was a feasible means of increasing sleep duration, a different course of treatment or preventative measures for hypertension or other cardiometabolic disease risk factors could be a more useful focus for this particular population. As mentioned earlier, circadian interventions focusing on sleep regularity over duration is an emerging area of research requiring more study. The advancement of knowledge and potential interventions for sleep and cardiometabolic health is crucial to promoting patient-provider discussions and increased focus on these intertwined aspects of health, particularly due to the high prevalence of issues in these areas and because during medical interviews, patients often fail to report receiving insufficient sleep, posing another obstacle to addressing health issues completely and comprehensively.[24]
One limitation of the current project is the lack of consideration for additional covariates that may affect metabolism such as food intake and timing. Though the parent study is collecting data on these variables, they have not been tabulated at the time of the current project. However, a linear mixed-effects regression model of body weight by segment adjusted for sex did not reveal any significant differences between weight at baseline and extension, suggesting that the effect of food intake may have been limited or remained fairly consistent between baseline and extension. A continuance of the current project could confirm whether this variable differed between the two segments.
Another limitation of the current project is the small sample size included in the analysis. Both glucose and insulin data as well as the covariate MVPA data were missing for some participants, restricting the number of observations included in both models 1 and 2. In model 2 in particular, many MVPA data points were missing, causing many observations to be excluded and producing singular fit errors for systolic blood pressure and fasting glucose analyses. Furthermore, as potential effects of sleep duration on CRP have been shown to vary by race, a much larger-scale project both in size and diversity would be required to adequately analyze CRP, particularly for any future project in populations with higher risk of elevated CRP.
References
1. Watson NF, Badr MS, Belenky G, et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. Jun 1 2015;38(6):843-4. doi:10.5665/sleep.4716
2. Pankowska MM, Lu H, Wheaton AG, et al. Prevalence and Geographic Patterns of Self-Reported Short Sleep Duration Among US Adults, 2020. Prev Chronic Dis. Jun 29 2023;20:E53. doi:10.5888/pcd20.220400
3. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. Feb 2010;33(2):414-20. doi:10.2337/dc09-1124
4. Eckel RH, Depner CM, Perreault L, et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Curr Biol. Nov 16 2015;25(22):3004-10. doi:10.1016/j.cub.2015.10.011
5. Broussard JL, Wroblewski K, Kilkus JM, Tasali E. Two Nights of Recovery Sleep Reverses the Effects of Short-term Sleep Restriction on Diabetes Risk. Diabetes Care. Mar 2016;39(3):e40-1. doi:10.2337/dc15-2214
6. Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep. May 1 2015;38(5):707-15. doi:10.5665/sleep.4660
7. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. Jun 2011;32(12):1484-92. doi:10.1093/eurheartj/ehr007
8. Petrie JR, Guzik TJ, Touyz RM. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can J Cardiol. May 2018;34(5):575-584. doi:10.1016/j.cjca.2017.12.005
9. Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. Jan-Feb 2009;51(4):294-302. doi:10.1016/j.pcad.2008.10.003
10. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. Mar 5 2002;105(9):1135-1143. doi:10.1161/hc0902.104353
11. Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. Aug 21 2003;92(4B):17K-22K. doi:10.1016/s0002-9149(03)00774-4
12. Chiang JK. Short duration of sleep is associated with elevated high-sensitivity C-reactive protein level in Taiwanese adults: a cross-sectional study. J Clin Sleep Med. Jul 15 2014;10(7):743-9. doi:10.5664/jcsm.3862
13. Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. May 1 2013;36(5):769-779E. doi:10.5665/sleep.2646
14. Farmer HR, Slavish DC, Ruiz J, et al. Racial/ethnic variations in inflammatory markers: exploring the role of sleep duration and sleep efficiency. J Behav Med. Dec 2022;45(6):855-867. doi:10.1007/s10865-022-00357-8
15. van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. doi:10.1371/journal.pone.0004589
16. Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. Feb 18 2004;43(4):678-83. doi:10.1016/j.jacc.2003.07.050
17. Leng Y, Ahmadi-Abhari S, Wainwright NW, et al. Daytime napping, sleep duration and serum C reactive protein: a population-based cohort study. BMJ Open. Nov 11 2014;4(11):e006071. doi:10.1136/bmjopen-2014-006071
18. Cagnacci A, Arangino S, Renzi A, et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf). Mar 2001;54(3):339-46. doi:10.1046/j.1365-2265.2001.01232.x
19. Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. Oct 1 2014;37(10):1715-9. doi:10.5665/sleep.4088
20. Huang T, Redline S. Cross-sectional and Prospective Associations of Actigraphy-Assessed Sleep Regularity With Metabolic Abnormalities: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care. Aug 2019;42(8):1422-1429. doi:10.2337/dc19-0596
21. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. Jan 2013;3(1):1-58. doi:10.1002/cphy.c110062
22. Taylor BJ, Matthews KA, Hasler BP, et al. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep. Feb 1 2016;39(2):457-65. doi:10.5665/sleep.5464
23. Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond). Oct 2013;125(7):311-8. doi:10.1042/CS20130140
24. Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare (Basel). Dec 20 2018;7(1)doi:10.3390/healthcare7010001
Acknowledgements
The parent study “Biomarkers and Altered Metabolic Pathways during Sleep Loss” from which the data for the current project were collected was funded by the National Institutes of Health through grants NIHK01HL145099 and NIHUM1TR004409 as well as by the Sleep Research Society Foundation and the University of Utah Office for Vice President of Research. The current project was supported by UROP from the Office of Undergraduate Research at the University of Utah awarded to Alisha Chong as well as by the Health Science LEAP program. Dr. Christopher Depner contributed valuable time, guidance, and education on sleep and cardiometabolic health topics for this project, and the project would not have been possible without his direction. Many thanks also to Michelle Kubicki, Grace Zimmerman, as well as all the members of the Sleep and Circadian Physiology Lab for their roles in collecting and analyzing data for the study and for all the help and advice they have provided.

