Transduction by Ionotropic Receptors
Caleb Bevan
Objective 2: Give examples of signal transduction by ionotropic receptors (ligand-gated ion channels).
A surprisingly small number of receptors are ligand-gated channels (ionotropic receptors). In this objective, we will look at two of the best-studied ionotropic receptors: the nicotinic acetylcholine receptor (nAChR) and the GABAA receptor.
The Nicotinic Acetylcholine Receptor
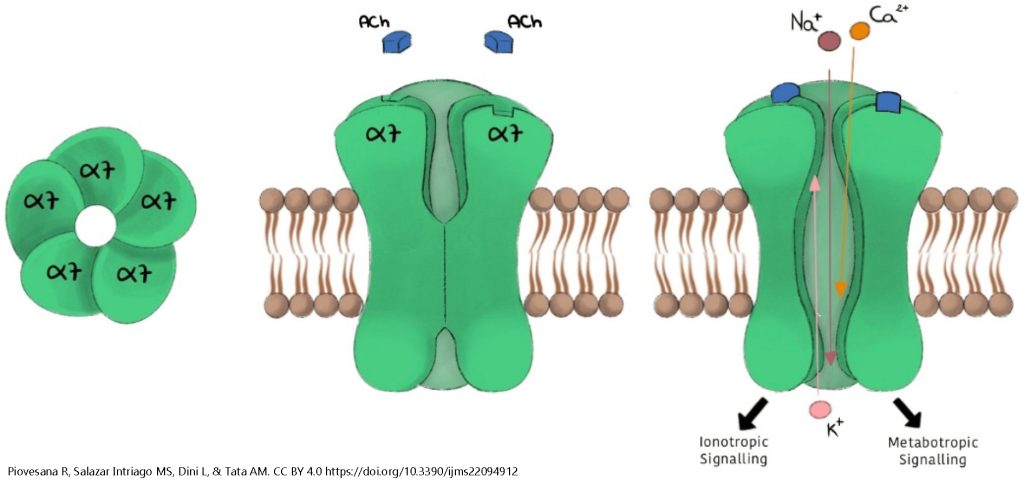
The nicotinic acetylcholine receptor always consists of five subunits, but the exact amino acid sequence varies for each of those receptors. There are five main gene products, designated α, β, γ, δ, and ε (the first five letters of the Greek alphabet). For example, the fetal muscle cell expresses the ααβγδ pentamer (five-part receptor) while adult muscle cells express the ααβδε pentamer. The brain expresses a number of different nAChR combinations, including:
- three α4 and two β2 [designated (α4)3(β2)2]
- two α4 and three β2 [(α4)2(β2)3]; and
- five α7 [(α7)5], shown here.
(The number indicates a slightly different form of the α or β protein.)
There is one ligand binding site per α subunit. When a ligand (acetylcholine, nicotine, or another drug that mimics acetylcholine) binds to the α subunit, the shape of the receptor protein changes, opening a channel for the ions Na+, Ca2+, and K+. Na+ and Ca2+ enter the neuron while K+ leaves. The net effect is to move the membrane potential close to 0 mV (i.e. about halfway between the equilibrium potential of Na+ and the equilibrium potential of K+). This is a large depolarization from the typical resting potential of –70 mV and is typically enough to stimulate an action potential or activate voltage-gated Ca2+ channels at the active zone of a synapse.
The GABAA Receptor
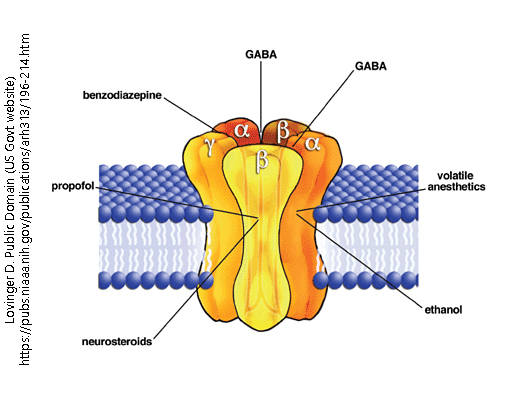 The GABAA receptor is the most common inhibitory neurotransmitter receptor in the human brain. Like all ionotropic receptors, it is a ligand-gated ion channel. When activated by ligand binding, the five protein subunits (two α, two β, and one γ) change shape to allow the passage of chloride (Cl–) ions.
The GABAA receptor is the most common inhibitory neurotransmitter receptor in the human brain. Like all ionotropic receptors, it is a ligand-gated ion channel. When activated by ligand binding, the five protein subunits (two α, two β, and one γ) change shape to allow the passage of chloride (Cl–) ions.
Recall that the equilibrium potential of Cl– is generally well below threshold for most neurons. For the action potential, threshold is the voltage at which voltage-gated Na+ channels engage in an unstoppable positive feedback loop; enough Na+ enters through voltage-gated Na+ channels to open more voltage-gated Na+ channels, which lets more Na+ into the neuron, and so forth until the peak of the action potential is reached.
For synaptic release of neurotransmitter, a similar threshold is reached where the entry of Ca2+ depolarizes the active zone to the point where the process becomes irreversible.
If we allow enough Cl– into the neuron that the membrane potential is held at or near ECl (the equilibrium potential for Cl–, which is generally near the resting potential), it becomes difficult for the neuron to reach threshold. Neuroscientists say that the membrane potential is “clamped” at ECl and cannot rise to a threshold potential. This means that when the GABAA receptor is open, and Cl– ions can move through the cell membrane, that the neuron is inhibited.
Media Attributions
- Nicotinic acetylcholine receptor alpha7 © Piovesana, Roberta, Michael Sebastian Salazar Intriago, Luciana Dini, and Ada Maria Tata is licensed under a CC BY (Attribution) license
- GABA receptor © David M. Lovinger is licensed under a Public Domain license

