The Molecules of Memory
Caleb Bevan
Objective 4: Summarize the molecular-level elements of a modifiable (Hebbian) synapse.
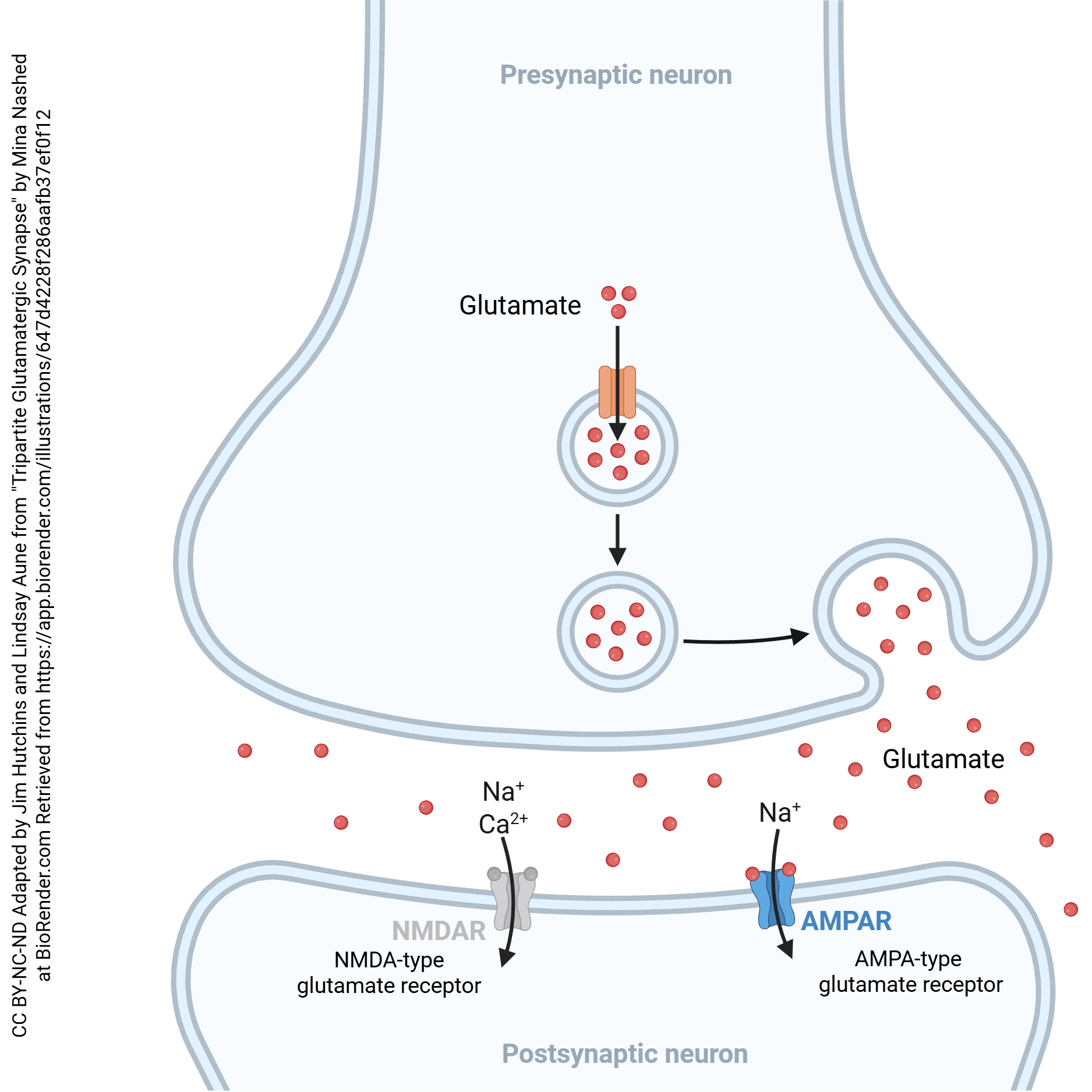 We’ll start our examination of modifiable synapses with a quick reminder about the parts of a synapse that are relevant to our discussion. The glutamatergic (glutamate-using) synapse is shown here, for reasons that will become clear later. The neurotransmitter released is the amino acid glutamate, which is packaged into vesicles. When voltage-gated calcium channels are activated by a superthreshold voltage change in the terminal, Ca2+ enters the synaptic terminal. Vesicles containing glutamate move to the presynaptic membrane and fuse, releasing glutamate.
We’ll start our examination of modifiable synapses with a quick reminder about the parts of a synapse that are relevant to our discussion. The glutamatergic (glutamate-using) synapse is shown here, for reasons that will become clear later. The neurotransmitter released is the amino acid glutamate, which is packaged into vesicles. When voltage-gated calcium channels are activated by a superthreshold voltage change in the terminal, Ca2+ enters the synaptic terminal. Vesicles containing glutamate move to the presynaptic membrane and fuse, releasing glutamate.
Glutamate diffuses across the synaptic cleft and binds to a glutamate receptor. Although there are many different kinds of glutamate receptor, some ionotropic and some metabotropic, we will focus on two of the ionotropic receptors: the AMPA receptor, which allows Na+ to flow into the cell, depolarizing it; and the NMDA receptor, which allows both Na+ and Ca2+ to flow into the postsynaptic neuron, also causing a depolarization but with the additional stimulus of a rise in intracellular Ca2+.
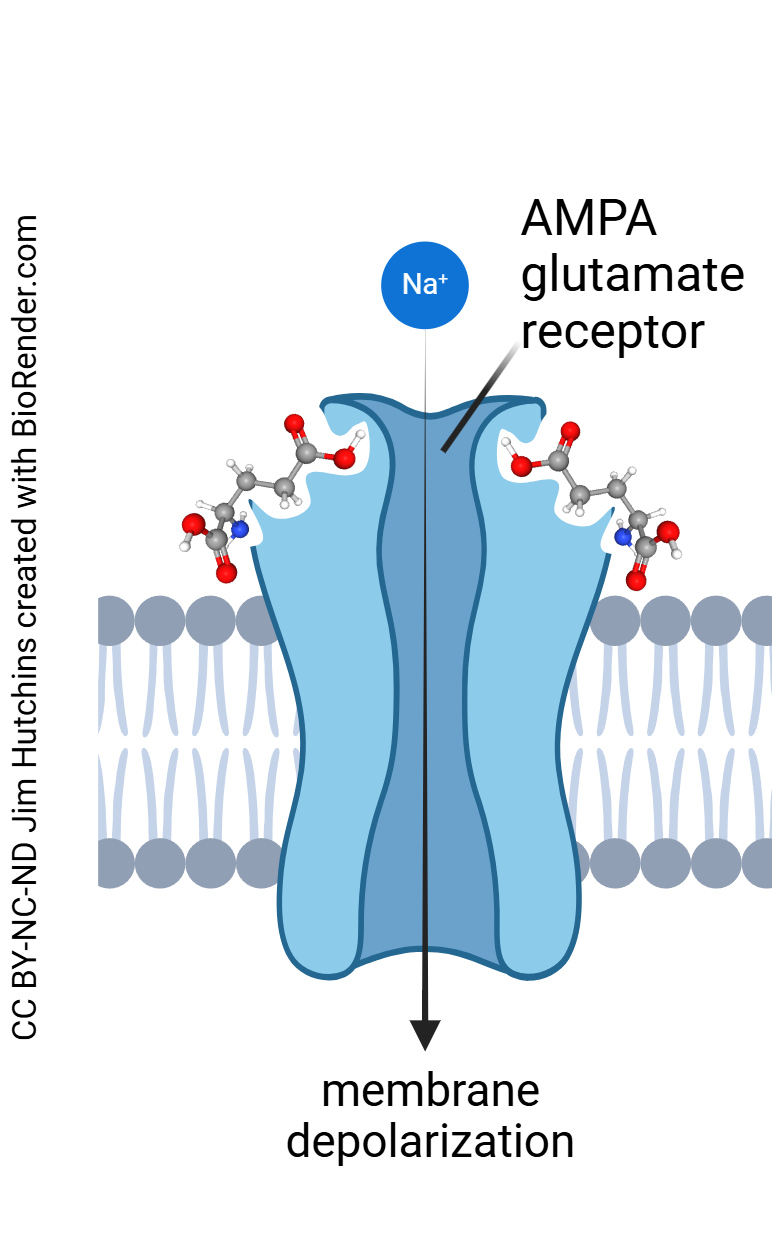
The AMPA Glutamate Receptor
Let’s take a detailed look at each of these receptors. The AMPA receptor is so named because it is maximally stimulated by the drug α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, which is thankfully abbreviated to the much-more-pronounceable “AMPA”. When glutamate binds to the AMPA receptor, the receptor protein changes shape, opening an ion channel which allows Na+ to flow into the postsynaptic neuron.
The NMDA Glutamate Receptor
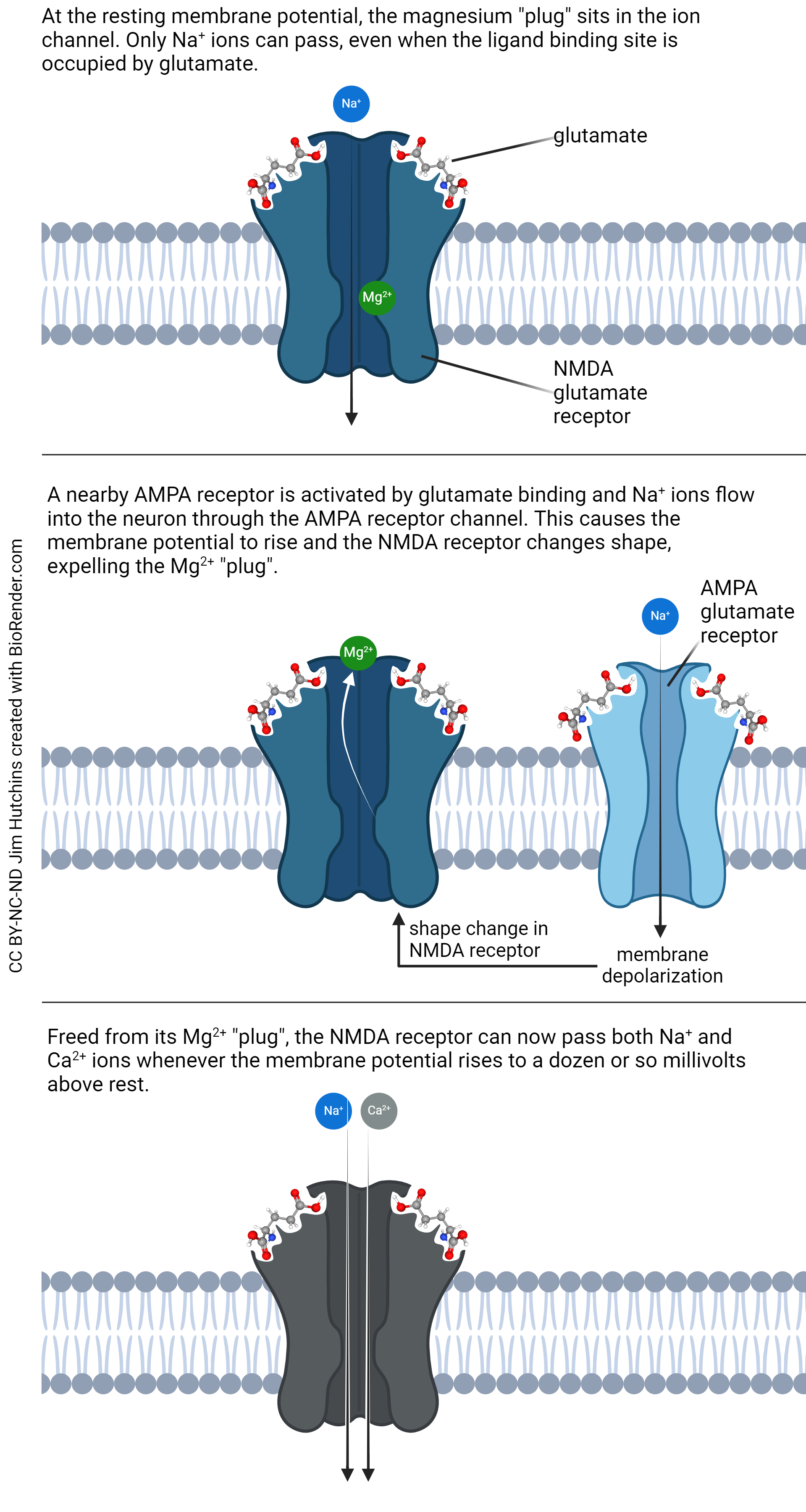
The N-methyl-D-aspartate (NMDA) receptor is much more complicated and therefore much more interesting. The NMDA receptor has two binding sites for glutamate. It also binds the drugs phencyclidine (PCP) and ketamine as well as the amino acid neurotransmitter glycine. These neurotransmitters and drugs all exert an inhibitory effect on the receptor, making it less likely to open.
If glutamate binds to the NMDA receptor at the resting potential, say –70 mV, only Na+ ions can flow. The channel contains a site for Mg2+, which plugs the channel in such a way that no other ions can flow. But a slight depolarization, say to –50 mV, causes a shape change in the protein that results in displacement of that Mg2+ plug. With the plug gone, now the channel permits the flow of both Na+ and Ca2+.
This change in channel permeability is important. Na+ “only” depolarizes the neuron, which is plenty important, but Ca2+ has many more effects on the postsynaptic neuron. Ca2+ ions set off a chain of biochemical events. Ca2+ ions bind to a small protein called calmodulin; together, Ca2+ ions and calmodulin activate an enzyme called calcium-calmodulin protein kinase II (CaMKII). Remember that a protein kinase adds phosphate groups onto a protein, changing its activity.
CaMKII phosphorylates a number of important protein kinases including protein kinase M zeta (PKMζ), which results in the creation of more “parking spaces” for AMPA receptors and which also anchors more AMPA receptors in those parking spaces, making the postsynaptic side of the synapse more sensitive to glutamate release.
(Yes, in this case a kinase is phosphorylating another kinase; this is a common sort of cascade in cell signaling. There is even a MAP kinase kinase kinase kinase but luckily we don’t have to worry about that one. It’s a lot like the Cat in the Hat Comes Back.)
CaMKII also decreases the activity of nitric oxide synthase, making less NO, which we mentioned earlier as an important retrograde signal.
In this section, I want to introduce three more players that will be important for an understanding of Objective 5, when we build a Hebbian synapse.
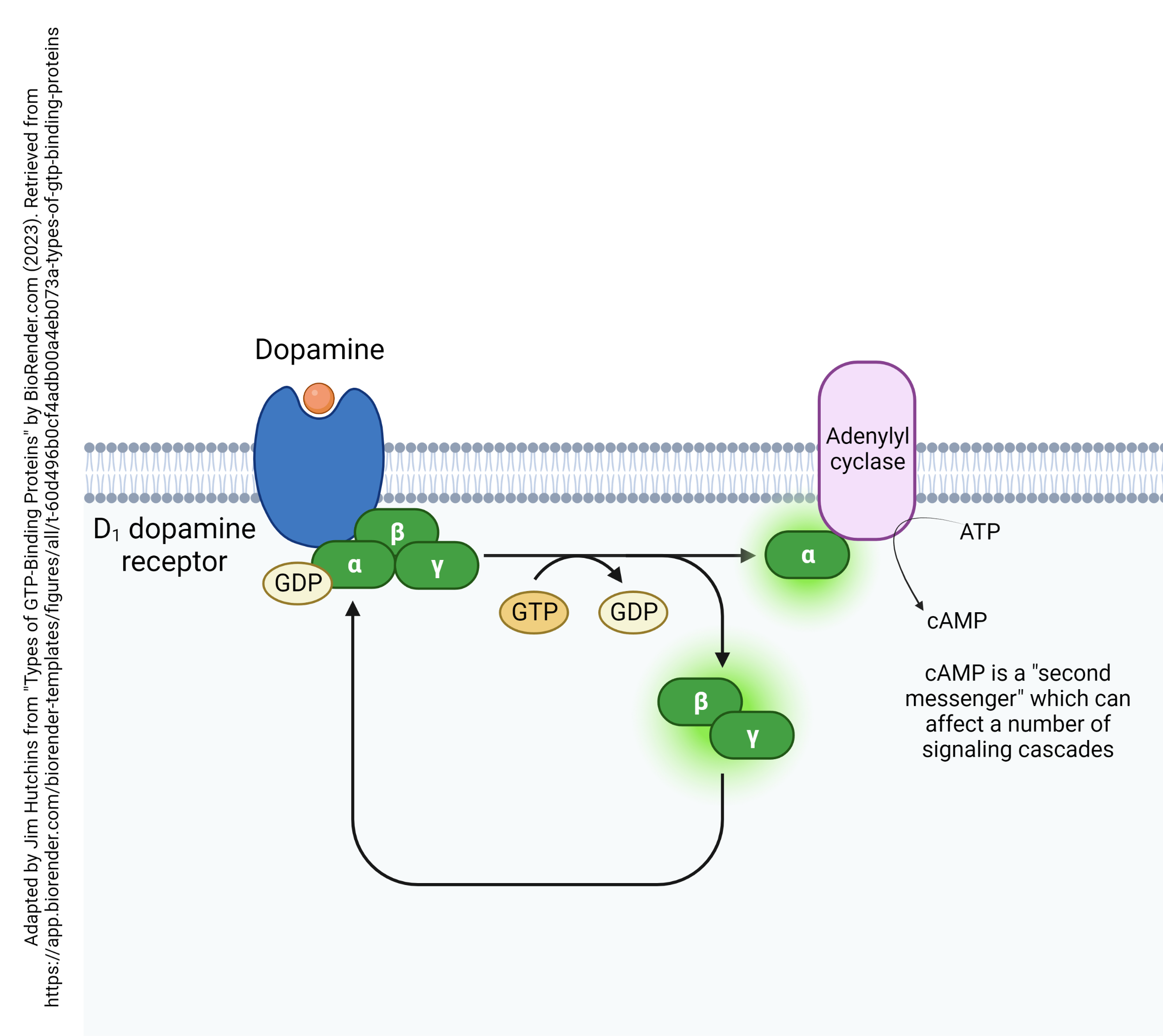
The D1 dopamine receptor is a G protein-coupled receptor attached to an αs subunit. Remember that this means binding of the D1 receptor releases a G protein α subunit that acts to stimulate adenylyl cyclase (adenylate cyclase) activity. Adenylyl cyclase then makes more cAMP, which activates a number of other signaling cascades.
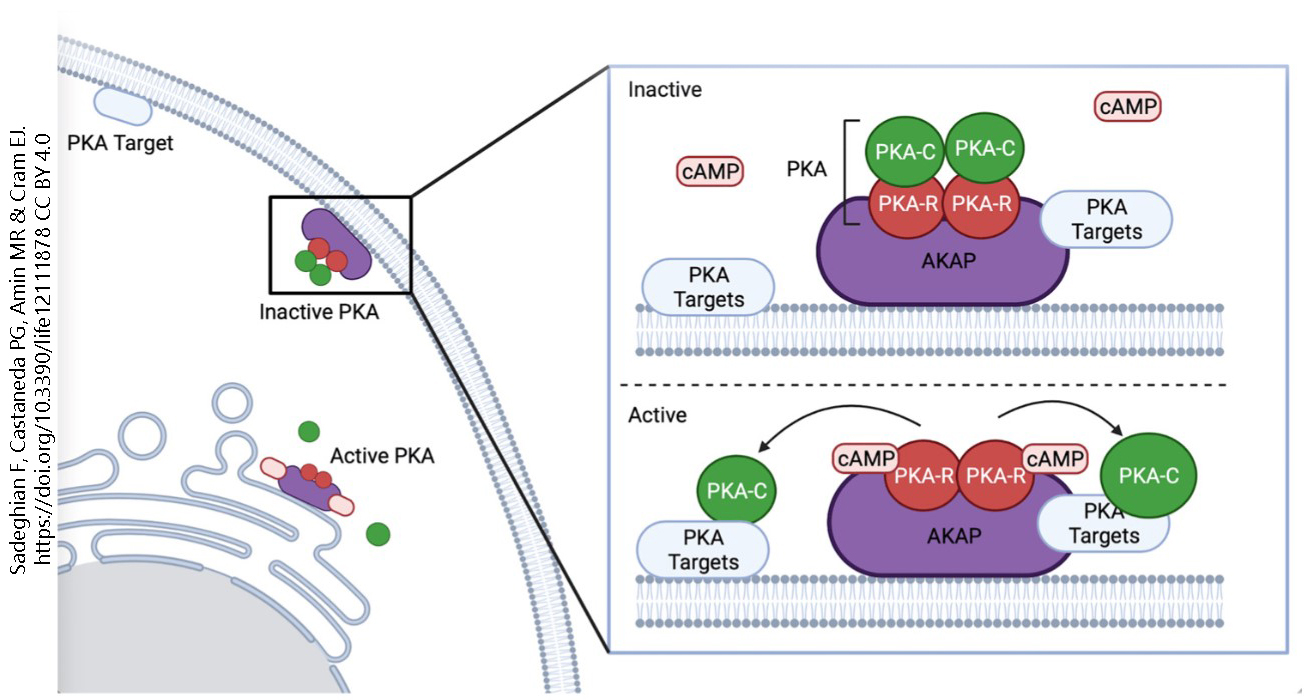
Protein kinase A comprises two regulatory subunits (PKA-R) and two catalytic subunits (PKA-C). Regulatory subunits bind cAMP, which turns on the enzyme; catalytic subunits catalyze the chemical reaction which is to add a phosphate group onto a protein. In the inactive form of the enzyme, two PKA-C and two PKA-R subunits are attached to an A kinase anchoring protein (AKAP). When cAMP binds to the regulatory (PKA-R) subunit, it is released, which exposes the catalytic site and allows the catalytic subunit (PKA-C) to act on whatever the enzyme is targeting (PKA targets).
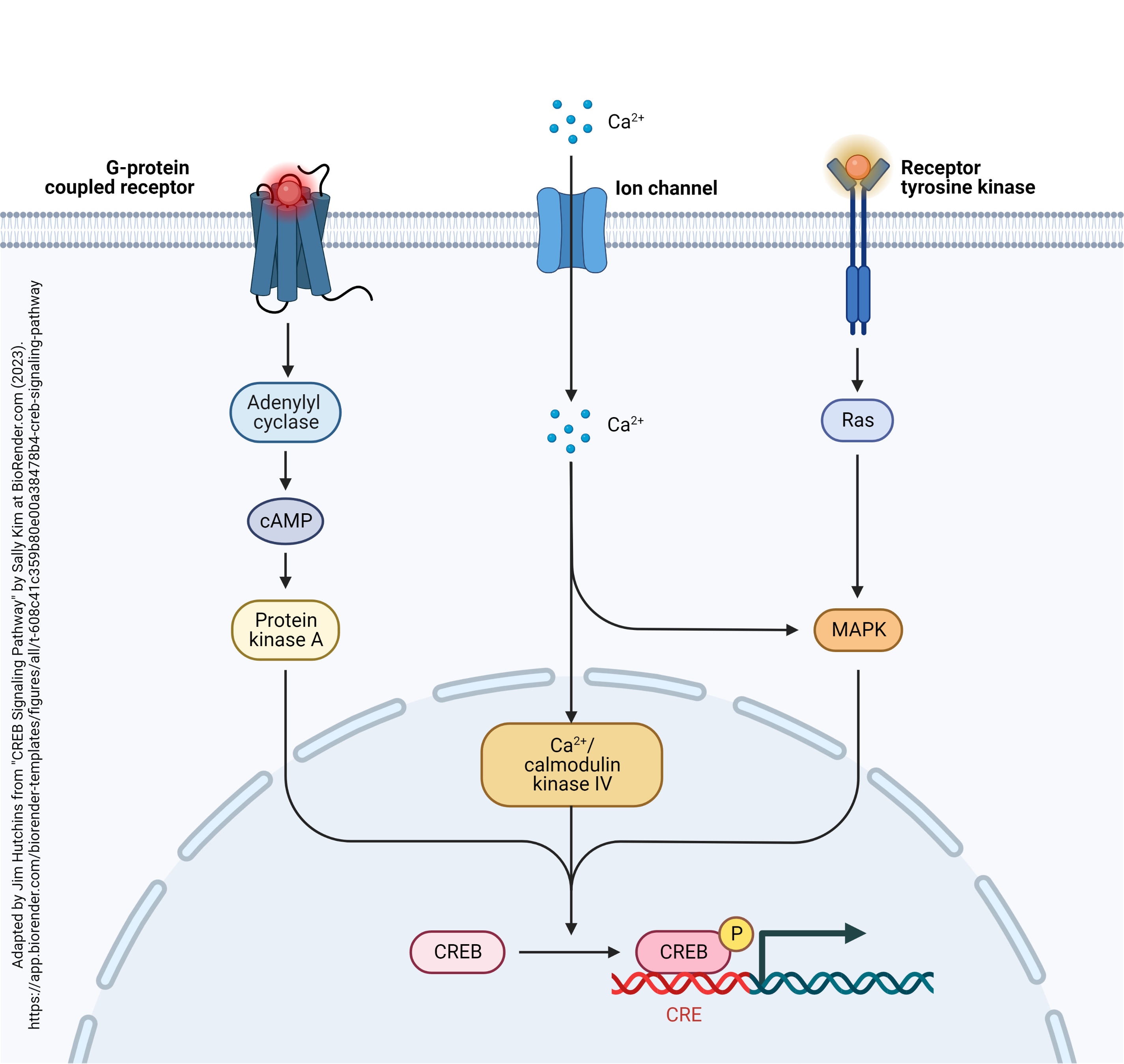 The ultimate goal of all this phosphorylation is to change gene expression. Remember that experimental evidence indicates that long-term potentiation outlasts the life cycle of any single neurotransmitter or protein molecule. There must be some alteration in a molecule that lasts for a lifetime. The only such molecule is DNA, and it’s changes in DNA expression that lie at the end of this road. Genes in DNA often contain a CREB-responsive element[1] (CRE). When the CRE-binding protein (CREB) is phosphorylated, it binds to the CRE, changing the transcription rate of the DNA downstream from the CRE.
The ultimate goal of all this phosphorylation is to change gene expression. Remember that experimental evidence indicates that long-term potentiation outlasts the life cycle of any single neurotransmitter or protein molecule. There must be some alteration in a molecule that lasts for a lifetime. The only such molecule is DNA, and it’s changes in DNA expression that lie at the end of this road. Genes in DNA often contain a CREB-responsive element[1] (CRE). When the CRE-binding protein (CREB) is phosphorylated, it binds to the CRE, changing the transcription rate of the DNA downstream from the CRE.
Media Attributions
- The Glutamatergic Synapse © Lindsay Aune and Mina Nashed adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- AMPA receptor © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- NMDA receptor and the Mg2+ plug © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Dopamine D1 receptor © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Protein kinase A © Fereshteh Sadeghian, Perla G. Castaneda, Mustafi R. Amin, and Erin J. Cram is licensed under a CC BY (Attribution) license
- CREB Signaling Pathway in Neurons © Sally Kim adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- The original name was cAMP response element, but it was changed by those who pointed out that the DNA sequence does not respond to cAMP directly. They wanted to keep the acronym so they changed it to the current CREB-responsive element which is circular, but there we are. ↵

