Signal Transduction
Caleb Bevan
Objective 1: Describe the salient features of signal transduction at the synapse.
The nervous system has the need to change energy from one form to another quite frequently. We will see in sensory systems how photons are transformed into an electrochemical signal in the retina, or how a fly landing on the skin is transformed into an electrical potential which travels down an axon to reach the spinal cord and beyond. In motor systems, electrical signals in motor neurons are turned into a chemical signal (release of acetylcholine) which is turned into an electrical signal which is turned back into a chemical signal (release of calcium ions) which is turned into movement of muscle proteins in contraction.
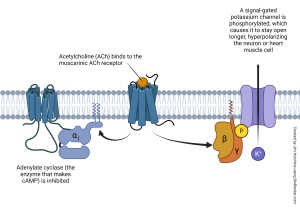 Signal transduction is the term of art neuroscientists use to describe conversion of energy from one form to another. At the chemical synapses we’ve seen so far, an electrical potential is transduced by voltage-gated calcium channels in the active zone of a synapse. Now the energy is in the form of vesicular fusion. As the vesicles fuse, they release chemical signaling molecules, and now the electrical signal has been transduced to a chemical signal. That chemical signal binds to a receptor. If the receptor is ionotropic, the signal is now electrical once again (i.e. the movement of electrically charged ions); if the receptor is metabotropic then the signal may retain its chemical form for a series of steps before being transduced to an electrical signal.
Signal transduction is the term of art neuroscientists use to describe conversion of energy from one form to another. At the chemical synapses we’ve seen so far, an electrical potential is transduced by voltage-gated calcium channels in the active zone of a synapse. Now the energy is in the form of vesicular fusion. As the vesicles fuse, they release chemical signaling molecules, and now the electrical signal has been transduced to a chemical signal. That chemical signal binds to a receptor. If the receptor is ionotropic, the signal is now electrical once again (i.e. the movement of electrically charged ions); if the receptor is metabotropic then the signal may retain its chemical form for a series of steps before being transduced to an electrical signal.
In the case of the muscarinic acetylcholine receptor which we examined previously, the βγ subunits of a G protein collide with a signal-gated potassium channel, causing it to gain a phosphate group which then means it’s open longer than it was before. The increased efflux of potassium causes the hyperpolarization of the muscle cell or neuron. Since it is more negative (closer to the potassium equilibrium potential), the neuron or muscle cell is less likely to fire an action potential. Inhibition is the name neuroscientists use for this type of response.
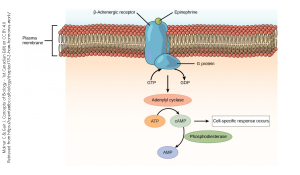 The chemical signal norepinephrine also acts through a G protein coupled receptor, but in the example shown here, the β-adrenergic receptor causes an increase in adenylyl cyclase activity, which in turn sets into motion a cascade of biochemical events that result in depolarization of a neuron or contraction of smooth muscle. These are classified as excitatory responses.
The chemical signal norepinephrine also acts through a G protein coupled receptor, but in the example shown here, the β-adrenergic receptor causes an increase in adenylyl cyclase activity, which in turn sets into motion a cascade of biochemical events that result in depolarization of a neuron or contraction of smooth muscle. These are classified as excitatory responses.
In each case, a chemical signal (acetylcholine; norepinephrine) has been transduced into an electrical signal.
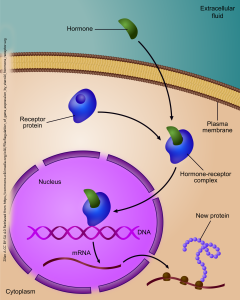 A form of transduction, from a chemical signal to a change in gene expression, is one mechanism by which neurosteroids act on the cells of the nervous system. In this case, the chemical signal does not act through a cell surface receptor, because steroids are capable of penetrating the non-polar region of the cell membrane without assistance.
A form of transduction, from a chemical signal to a change in gene expression, is one mechanism by which neurosteroids act on the cells of the nervous system. In this case, the chemical signal does not act through a cell surface receptor, because steroids are capable of penetrating the non-polar region of the cell membrane without assistance.
Rather, in this case, the receptor is found in the cytoplasm of the cell. Because the cytoplasm is water-based, and therefore polar, the non-polar, lipid-soluble hormone must associate with an intracellular receptor in order to move within the neuron. The hormone-receptor complex is carried through a nuclear pore into the nucleus, where it can act as a transcription factor that alters the course of gene expression. In this way, a chemical signal is transduced into a change in protein expression.
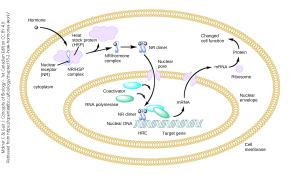 Heat-shock proteins are important intermediaries that help neurons (and other cells) respond to stressors in the environment, such as heat (hence the name), cold, ultraviolet light, or reactive oxygen species. The genes needed for the neuron to respond to these stressors are activated by the binding of a lipid-soluble chemical signal to an intracellular receptor which then is carried to the nucleus and causes changes in gene expression, leading to an alteration in cell function.
Heat-shock proteins are important intermediaries that help neurons (and other cells) respond to stressors in the environment, such as heat (hence the name), cold, ultraviolet light, or reactive oxygen species. The genes needed for the neuron to respond to these stressors are activated by the binding of a lipid-soluble chemical signal to an intracellular receptor which then is carried to the nucleus and causes changes in gene expression, leading to an alteration in cell function.
Media Attributions
- Muscarinic acetylcholine receptor © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Neurotransmitter GPCR transduction © Molnar, Charles and Gair, Jane is licensed under a CC BY-SA (Attribution ShareAlike) license
- Mechanism of steroid hormone transduction © Ali Zifan is licensed under a CC BY-SA (Attribution ShareAlike) license
- Lipid soluble hormone transduction heat shock protein © Boghog2 is licensed under a Public Domain license

