Neurotransmitter Release
Caleb Bevan
Objective 3: Explain how vesicles are fused with the cell membrane to release neurotransmitter.
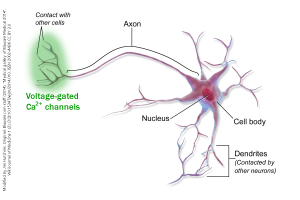 When a voltage change reaches the active zone of a synapse, it acts on the voltage-gated calcium (Ca2+) channels found there.
When a voltage change reaches the active zone of a synapse, it acts on the voltage-gated calcium (Ca2+) channels found there.
If the neuron is the typical or canonical type, forming axodendritic synapses onto a postsynaptic cell, then the voltage-gated Ca2+ channels are found only in the axon terminals.
Normally the calcium concentration of the presynaptic terminal is about 10,000X lower than the surrounding extracellular fluid. Opening a voltage-gated Ca2+ channel, then, will allow Ca2+ ions to rush into the presynaptic terminal. Ca2+ then binds to activator proteins, and in a complex biochemical cascade, the vesicle is dragged to the presynaptic membrane, fused with the membrane, and the vesicle contents are released into the synaptic cleft.
In a canonical neuron, such as the one shown here, voltage-gated Ca2+ channels are located in the axon terminals. But if the active zones are found on synapses in the neuronal cell body (soma), that obviously won’t work. So in the case of somatodendritic, somatosomatic, and somatoaxonal synapses, the voltage-gated Ca2+ channels would be found in the soma.
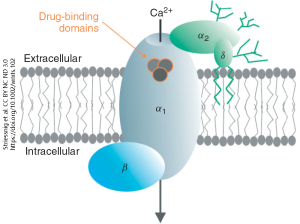
Voltage-gated Ca2+ channels are often the target of drug therapies, particularly in the heart where they play an important role in the rhythmic timing of heart muscle contraction. There are at least four protein pieces which make up the complete voltage-gated calcium channel. The pore that opens in response to a voltage change is in the alpha-1 (α1) subunit. The α2 subunit and delta (δ) subunit are attached to α1 on the outside surface of the neuron; a pair of lipid tails on the δ subunit anchor the whole complex into the cell membrane.
When the membrane voltage rises above the resting value, the α1 subunit changes shape, opening a channel and allowing Ca2+ ions to enter the presynaptic terminal.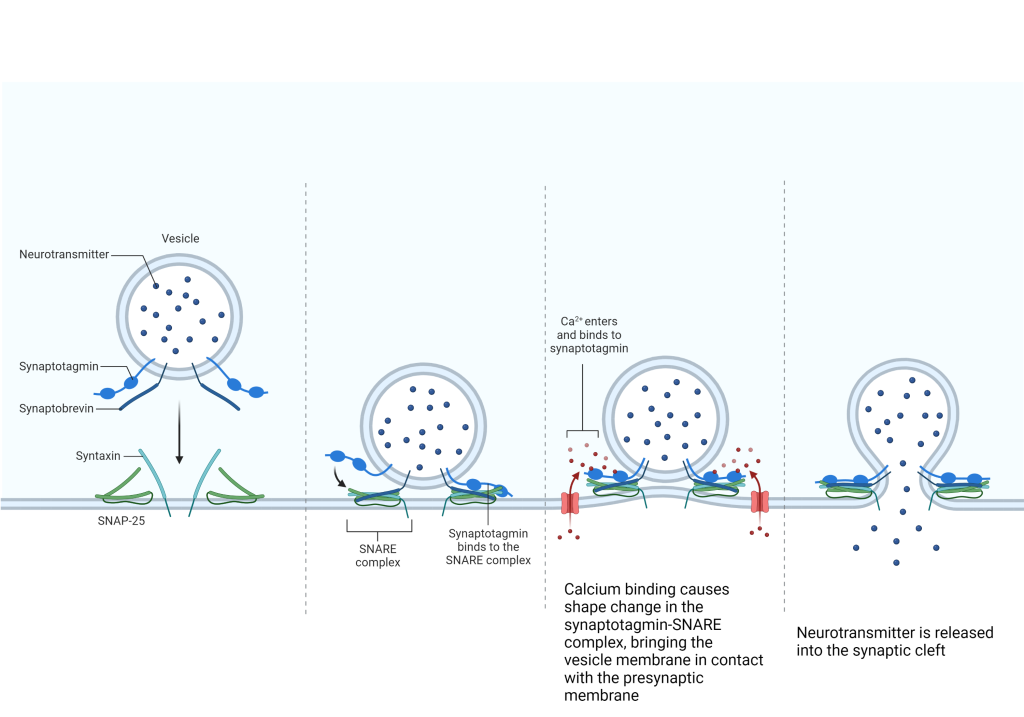 The mechanism by which Ca2+ entry is converted into transmitter release is not simple or straightforward. Vesicles are coated with a protein called synaptotagmin. Ca2+ binds synaptotagmin and the resulting shape change allows a nearby protein, synaptobrevin, to join with a protein of 25 kilodaltons (kDa) molecular weight called synaptosomal-associated protein (SNAP-25). SNAP-25 is part of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. When Ca2+ is bound to synaptotagmin, a chain reaction causes synaptobrevin to be snared by the whole SNARE complex followed by collapse of the SNARE-synaptobrevin complex. This collapse brings the vesicle into close proximity with the presynaptic membrane and fusion of the phospholipid bilayers of the vesicle and neuronal membrane occurs. The contents of the vesicle are now continuous with the extracellular fluid of the synaptic cleft, and neurotransmitter is free to diffuse across the small gap (20-30 nm, or about 250 hydrogen atoms wide).
The mechanism by which Ca2+ entry is converted into transmitter release is not simple or straightforward. Vesicles are coated with a protein called synaptotagmin. Ca2+ binds synaptotagmin and the resulting shape change allows a nearby protein, synaptobrevin, to join with a protein of 25 kilodaltons (kDa) molecular weight called synaptosomal-associated protein (SNAP-25). SNAP-25 is part of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. When Ca2+ is bound to synaptotagmin, a chain reaction causes synaptobrevin to be snared by the whole SNARE complex followed by collapse of the SNARE-synaptobrevin complex. This collapse brings the vesicle into close proximity with the presynaptic membrane and fusion of the phospholipid bilayers of the vesicle and neuronal membrane occurs. The contents of the vesicle are now continuous with the extracellular fluid of the synaptic cleft, and neurotransmitter is free to diffuse across the small gap (20-30 nm, or about 250 hydrogen atoms wide).
Now the neurotransmitter can find, and bind to, a receptor on the postsynaptic membrane. Neurotransmission has taken place.
Media Attributions
- Voltage gated calcium channel distribution neuron © BruceBlaus adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- Voltage-gated calcium channel © Jörg Striessnig, Alexandra Pinggera, Gurjot Kaur, and Gabriella Bock is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Mechanism of vesicle fusion at the presynaptic membrane © BioRender is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

