Axonal Transport
Objective 5. Classify anterograde and retrograde axonal transport mechanisms.
Neuronal Energy Objective 5 Video Lecture
Types of Axoplasmic Transport
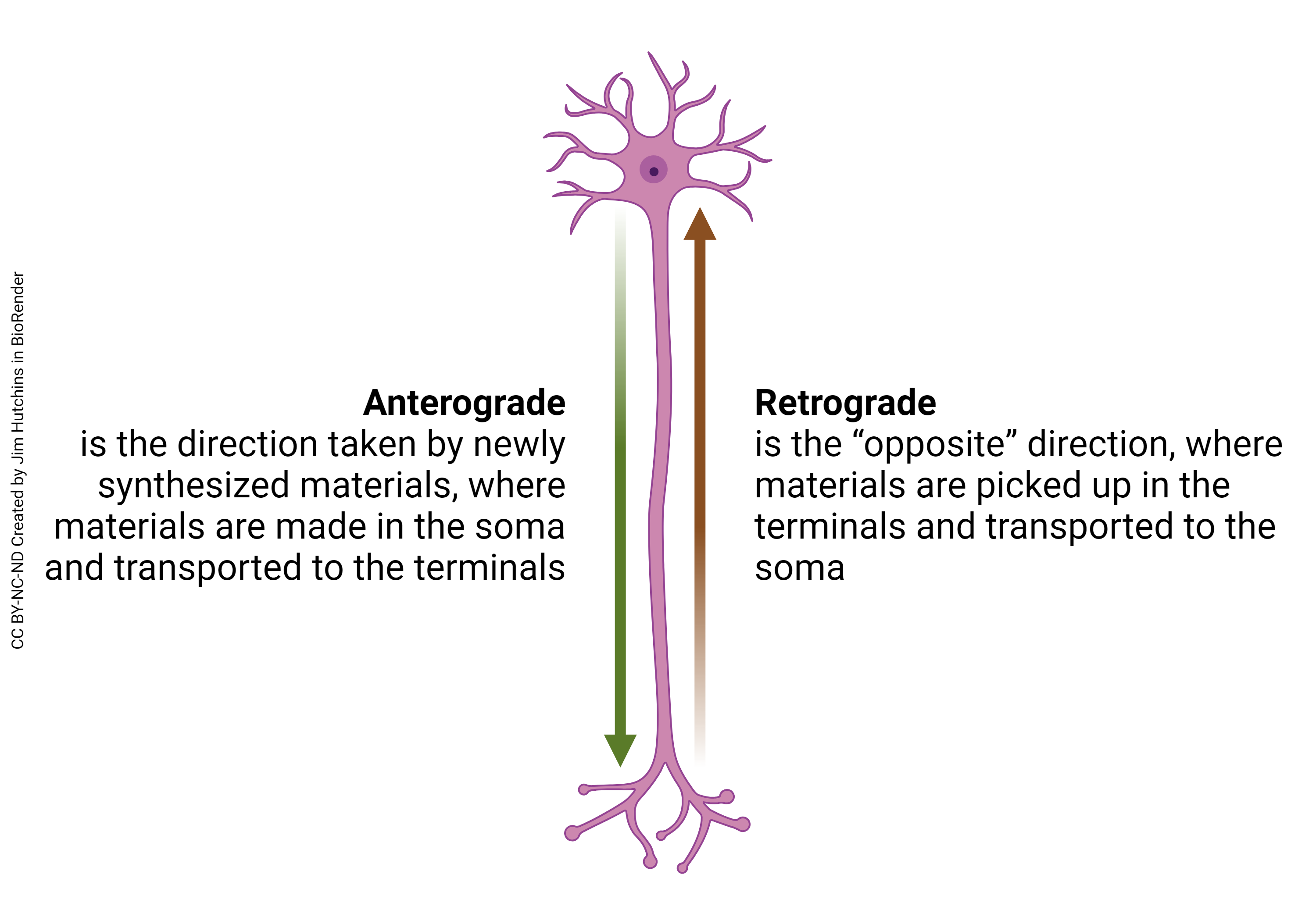
Another problem that must be solved by neurons is how to get substances from the cell body, where they are made, to the axon terminals. Returning to the football analogy, if we scaled up the cell body to the size of a football, we would have to send material through a drinking straw over a distance equal to a marathon.
Neurons also need to receive signals from the axon terminal that tell the cell body how it’s going. For example, the mitochondria may be depleted and need to be replaced. More enzymes for neurotransmitter synthesis may be needed. The axon terminal can communicate alerts about these crises by sending a signal back to the cell body.
The direction of material or information transfer which goes from the cell body to the axon terminal (i.e. the “forward” direction) is called anterograde.
The direction of material or information transfer which goes from the axon terminal to the cell body (i.e. the “backward” direction) is called retrograde.
Rates of Axonal Transport
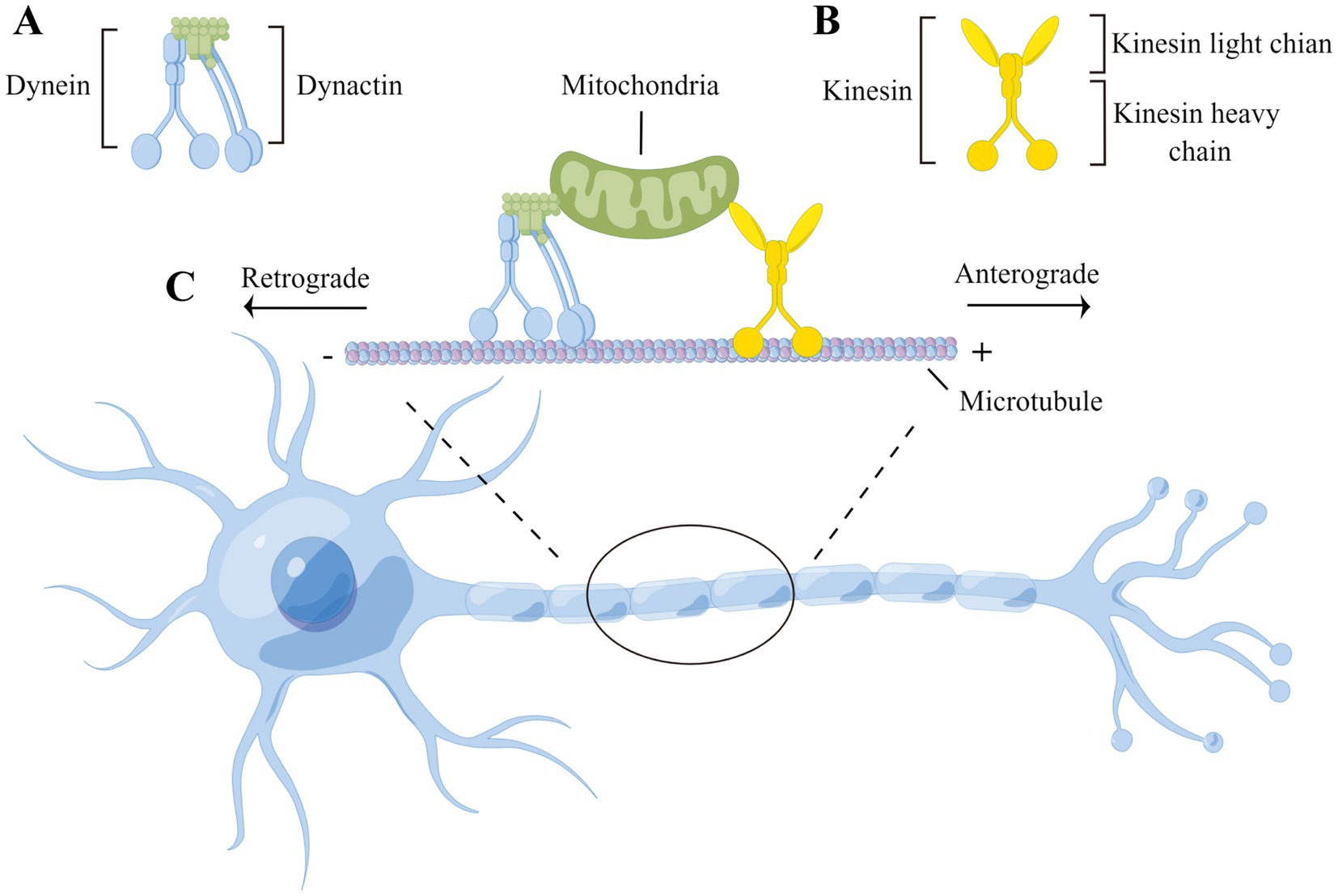
Although there are exceptions, in general, anterograde transport is mediated by the protein kinesin. Kinesin is shaped like a headless person, with “shoulders” that carry a cargo (such as a vesicle) and “legs” that “walk” along a microtubule. Microtubules have a “plus” end oriented toward the axon terminal and a “minus” end oriented toward the cell body. Thus, kinesin carries in the minus to plus direction along the microtubule. Although an animation of this process is kinda ridiculous looking, it is an actual representation of the conformational changes in kinesin that result in anterograde axonal transport.
Dynein and dynactin are the corresponding proteins that move organelles from the plus end to the minus end of the microtubule and therefore carry cargo in the retrograde direction.
Dynein’s conformational change results in a motion that Harvard Medical School describes as “like a drunken sailor”.
We can see the smooth transition between the protein synthesis system, the endomembrane system, and axoplasmic transport in how transmitters made from strings of amino acids (neuropeptides) are loaded into the axon terminals of peptidergic neurons.
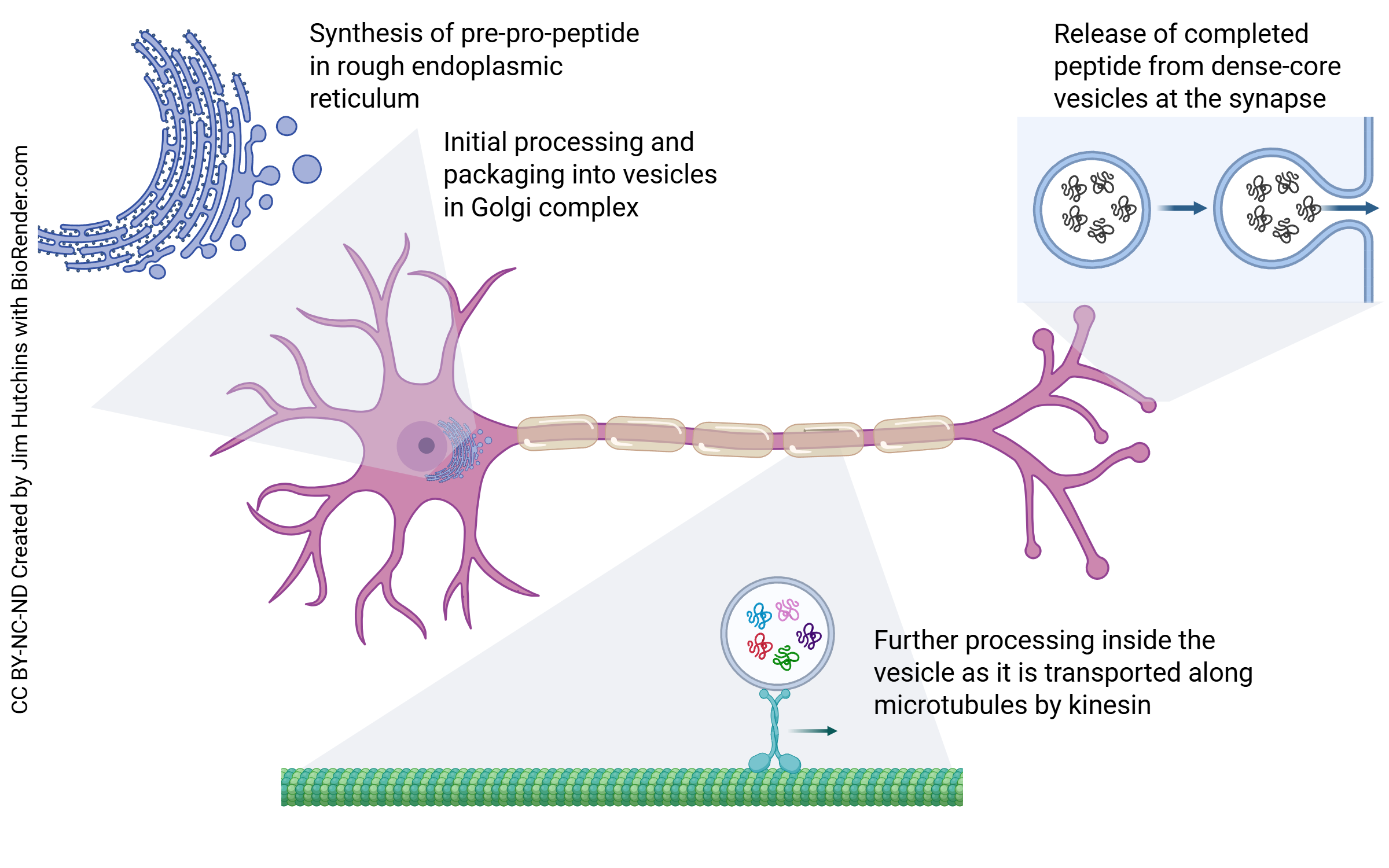 Peptide neurotransmitters are made as a pre-pro-peptide, which has a small sequence which is kept but a much larger piece that is discarded. An example is the peptide neurotransmitter β-endorphin, which is a small piece of the pre-pro-opiomelanocortin peptide.
Peptide neurotransmitters are made as a pre-pro-peptide, which has a small sequence which is kept but a much larger piece that is discarded. An example is the peptide neurotransmitter β-endorphin, which is a small piece of the pre-pro-opiomelanocortin peptide.
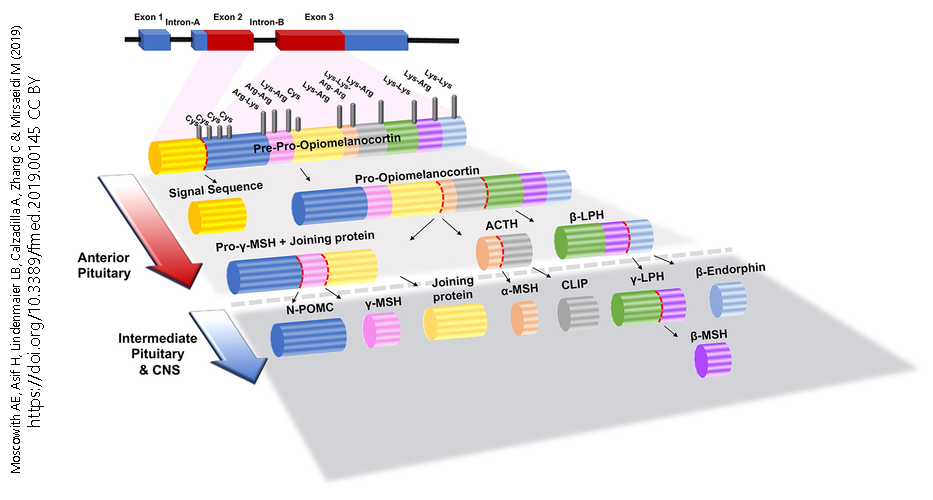
As we will see in the molecular biology section, most genes consist of exons which are expressed and introns or intervening regions which are discarded. The first step is creation of an RNA molecule, the primary transcript, which is a direct, letter by letter copy of the DNA gene. The primary transcript is edited to form the messenger RNA (mRNA). The mRNA is transported from the nucleus to the rough endoplasmic reticulum where it is translated into a sequence of amino acids (i.e. a peptide). In the Golgi, the signal sequence is removed to make pro-opiomelanocortin (POMC). As this pro-peptide is moved through the Golgi, a vesicle containing enzymes that cleave the peptide are added to the vesicle which is shed from the trans face of the Golgi.
This vesicle, which contains both the peptide to be processed and the processing enzymes that work on it, is transported by kinesin. As anterograde axonal transport proceeds over the next several days, the enzymes continue to work on the pro-opiomelanocortin cleaving it into pieces as shown above.
Finally, by the time it reaches the axon terminals, it is β-endorphin along with amino acids which are the breakdown products from the unused parts of the POMC peptide. The amino acids cannot bind to receptors on the receiving (postsynaptic) cell. They are taken back up by the presynaptic cell and used to make other peptides.
Media Attributions
- Anterograde vs Retrograde Axoplasmic Transport © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Axonal transport © Xioaman Yang, Zhuoran Ma, Piaopiao Lian, Yan Xu, and Xuebing Cao Xuebing is licensed under a CC BY (Attribution) license
- Axonal transport of neuropeptides © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Pre pro opiomelanocortin © AE Moscowitz, H Asif, LB Lindenmaier, A Calzadilla, C Zhang, and M Mirsaeidi is licensed under a CC BY (Attribution) license

