Energy Allocation
Jim Hutchins
Objective 2: Explain how neurons allocate their energy among various essential cell functions.
Neuronal Energy Objective 2 Video Lecture
 The neuron has an almost unmanageably huge surface area to maintain. If we were to scale a neuronal cell body (about 10 μm, or about 0.01 mm) up to the size of a football, then the surface area of a neuron (2000 mm2) would be the size of 250 football pitches.
The neuron has an almost unmanageably huge surface area to maintain. If we were to scale a neuronal cell body (about 10 μm, or about 0.01 mm) up to the size of a football, then the surface area of a neuron (2000 mm2) would be the size of 250 football pitches.
As we will see, the surface of a neuron carries all kinds of proteins which help maintain the electrical environment inside the cell. There is a huge difference in charged ions, such as K+, Na+, Ca2+, and Cl–, between the inside and the outside of the cell. These differences are maintained by leakage channels, antiport systems, symport systems, uniport systems, and pumps.
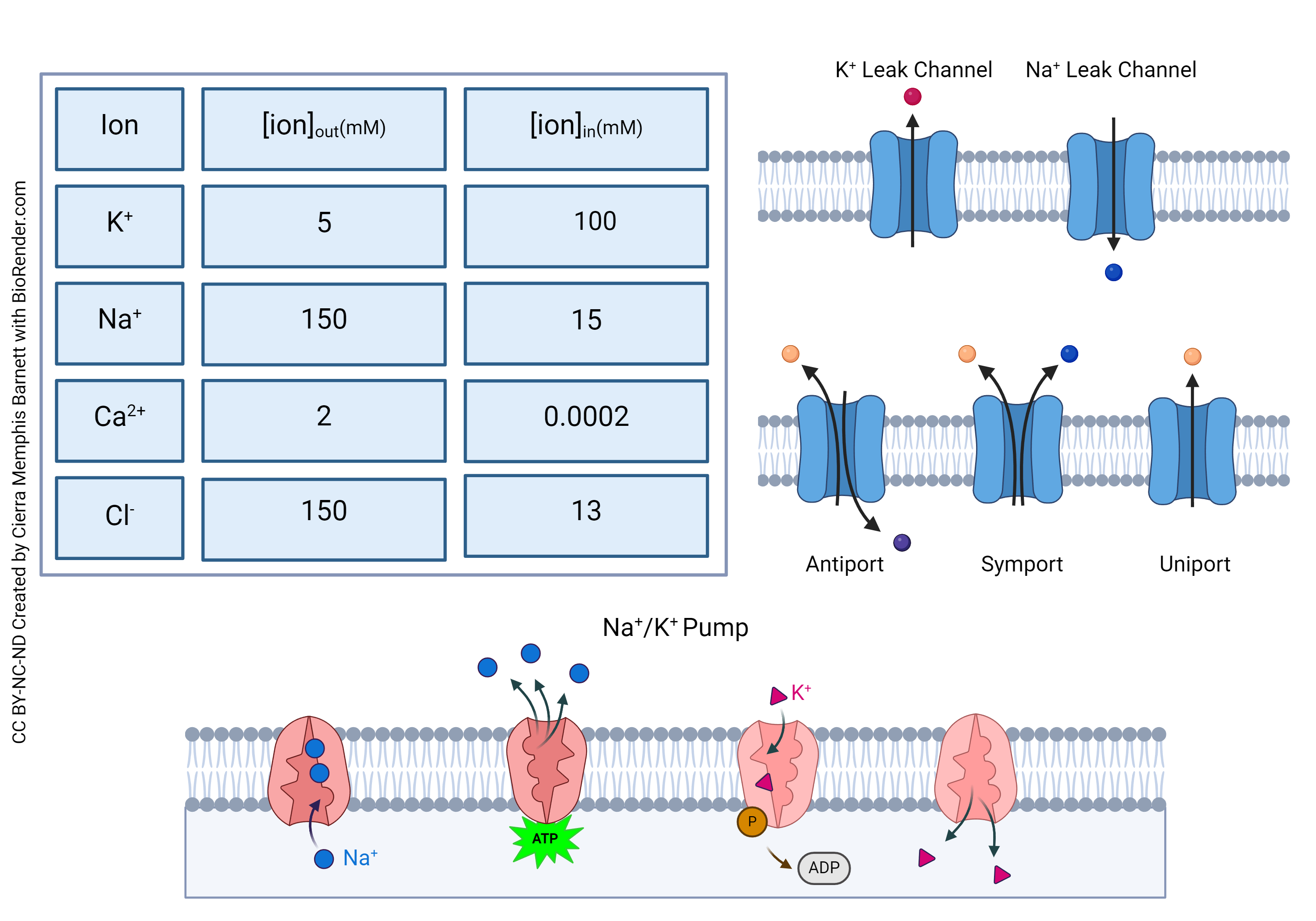 The sodium-potassium pump and leakage channels maintain the difference in potassium and sodium concentrations between the inside and outside of the neuron.
The sodium-potassium pump and leakage channels maintain the difference in potassium and sodium concentrations between the inside and outside of the neuron.
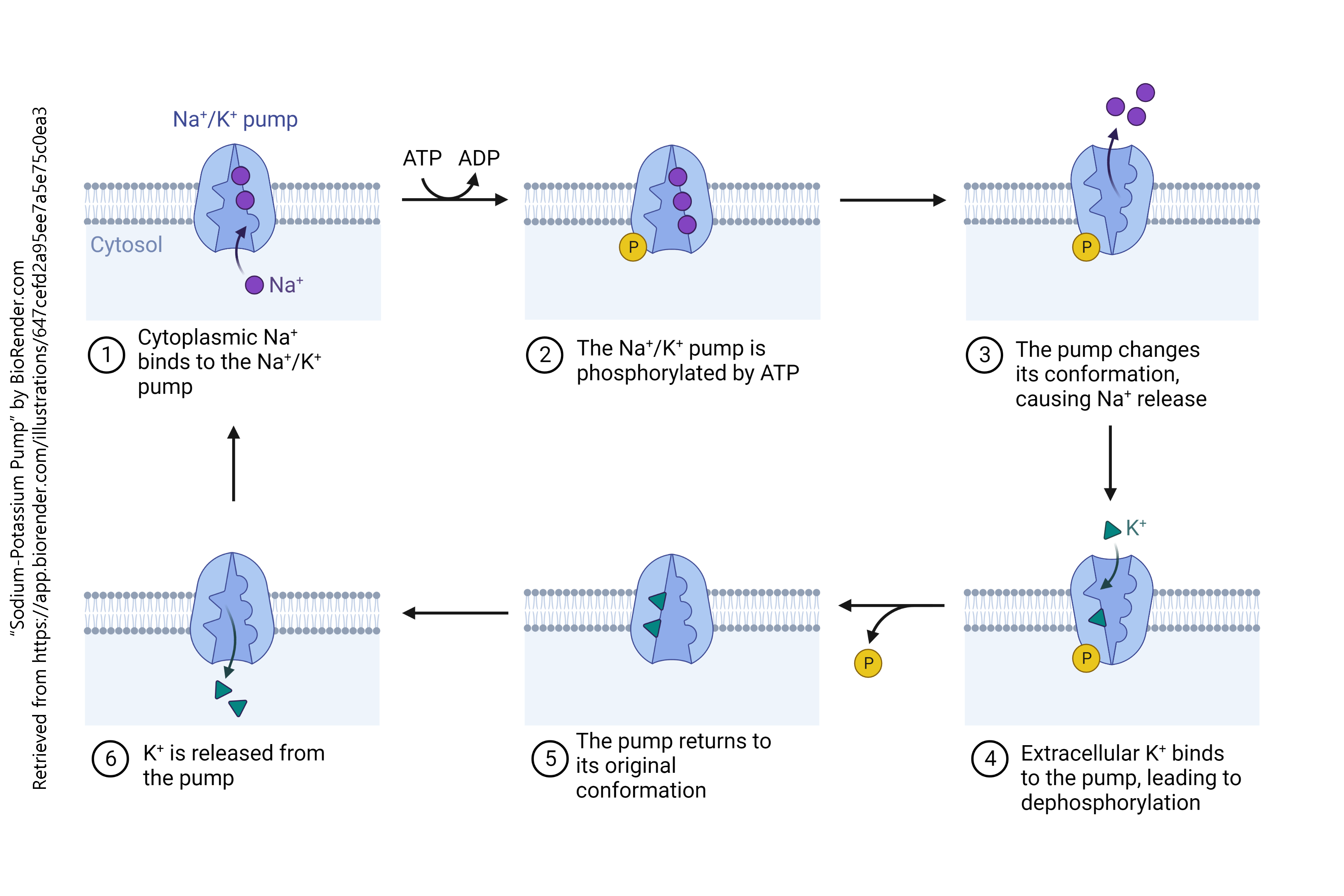
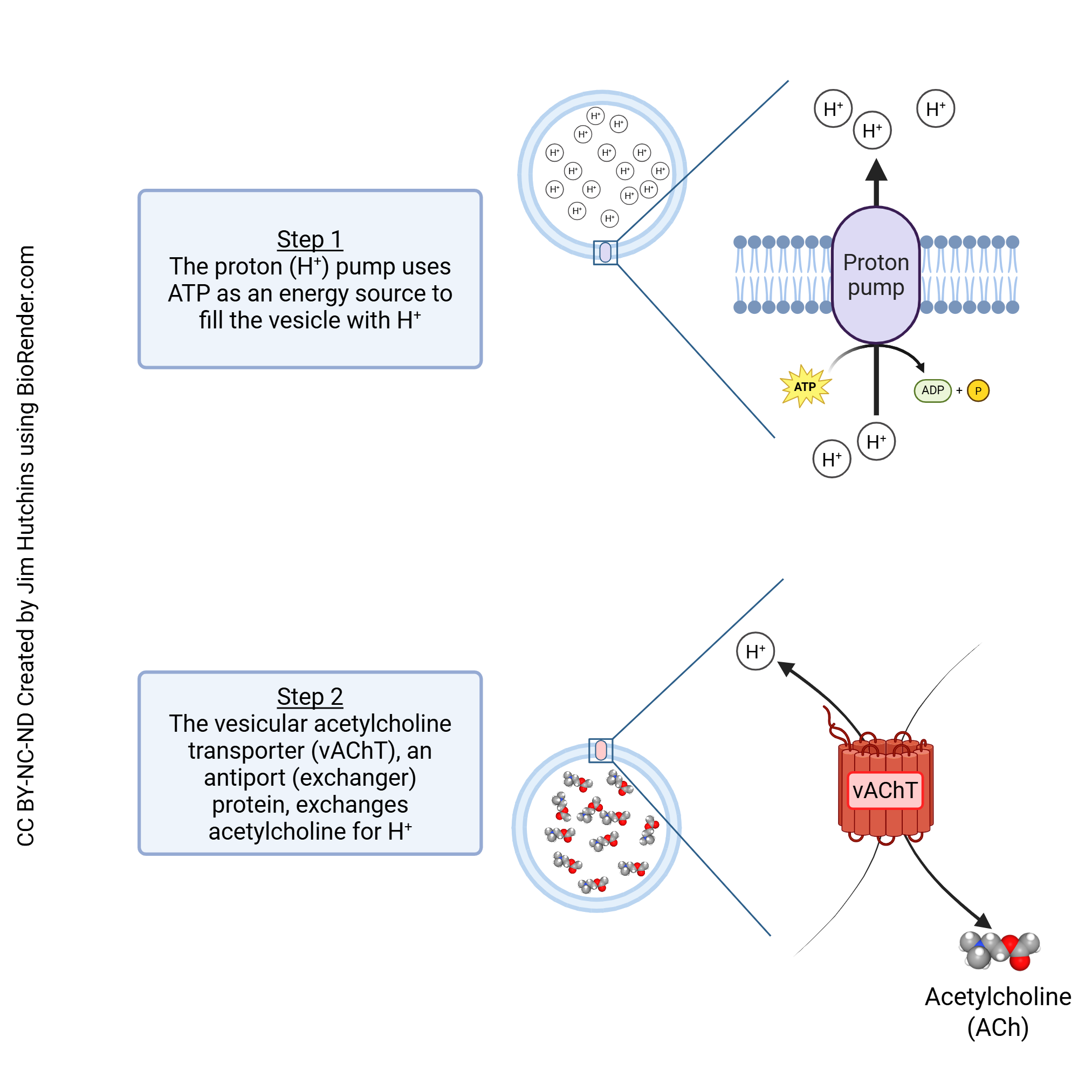
Neurotransmitters are packaged into vesicles using a combination of proton (H+) pumps and either symport or antiport system. The antiport system which is used to package acetylcholine into vesicles is shown here.
Taken together, then, neurons have huge energy needs. This is exemplified by the fact that the human brain, at 2% of body weight, receives 20% of the blood pumped by the heart and therefore consumes about 20% of the oxygen and glucose used by the entire body.
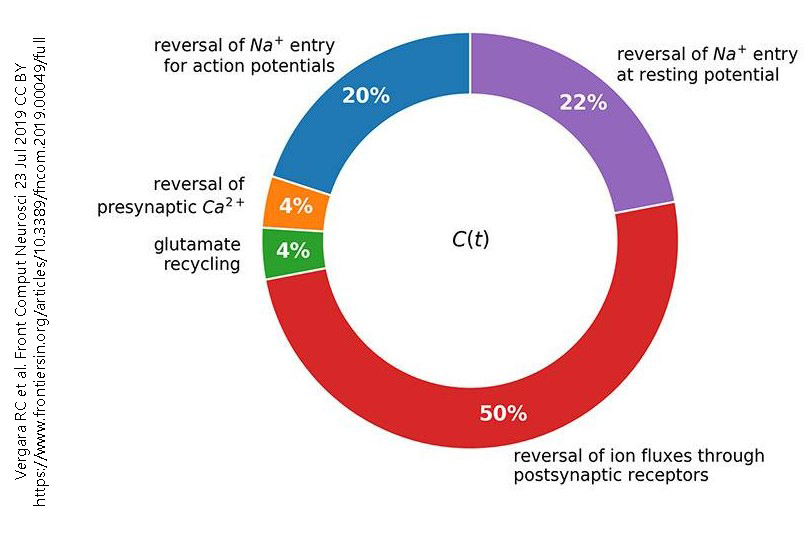
A summary of the estimated energy usage by neurons is shown in this pie chart. A breakdown of these energy requirements is given below.
Reversal of Ion Fluxes Through Postsynaptic Receptors
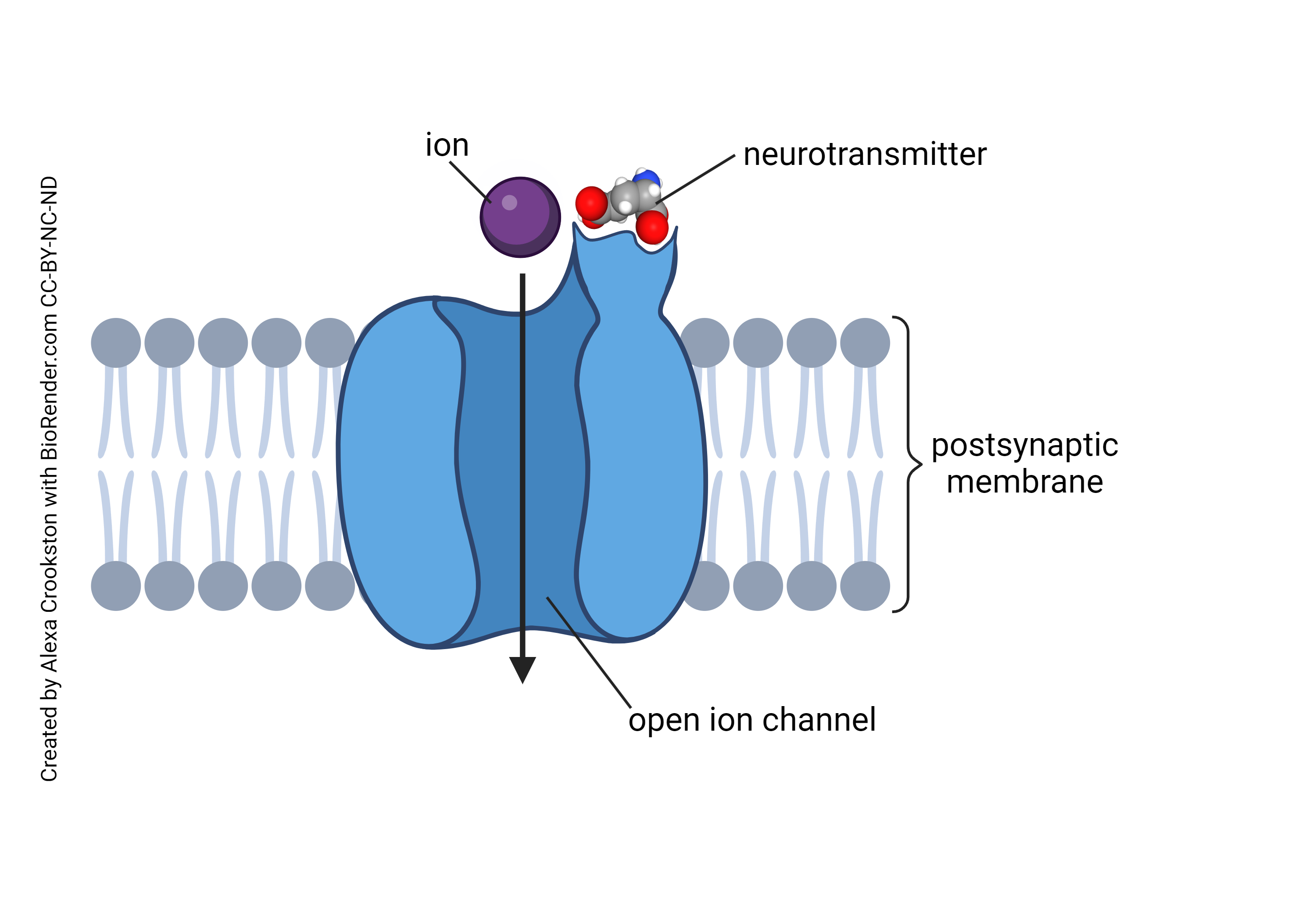
When neurotransmitter binds to an ionotropic receptor, there is a change in the flow of charged ions. Ions flow from where they are at high concentration to where they are at low concentration. It takes energy to restore these ions to their “proper” location, that is, to move them from where they are at low concentration to where they are at high concentration.
Reversal of Na+ Entry at Resting Potential
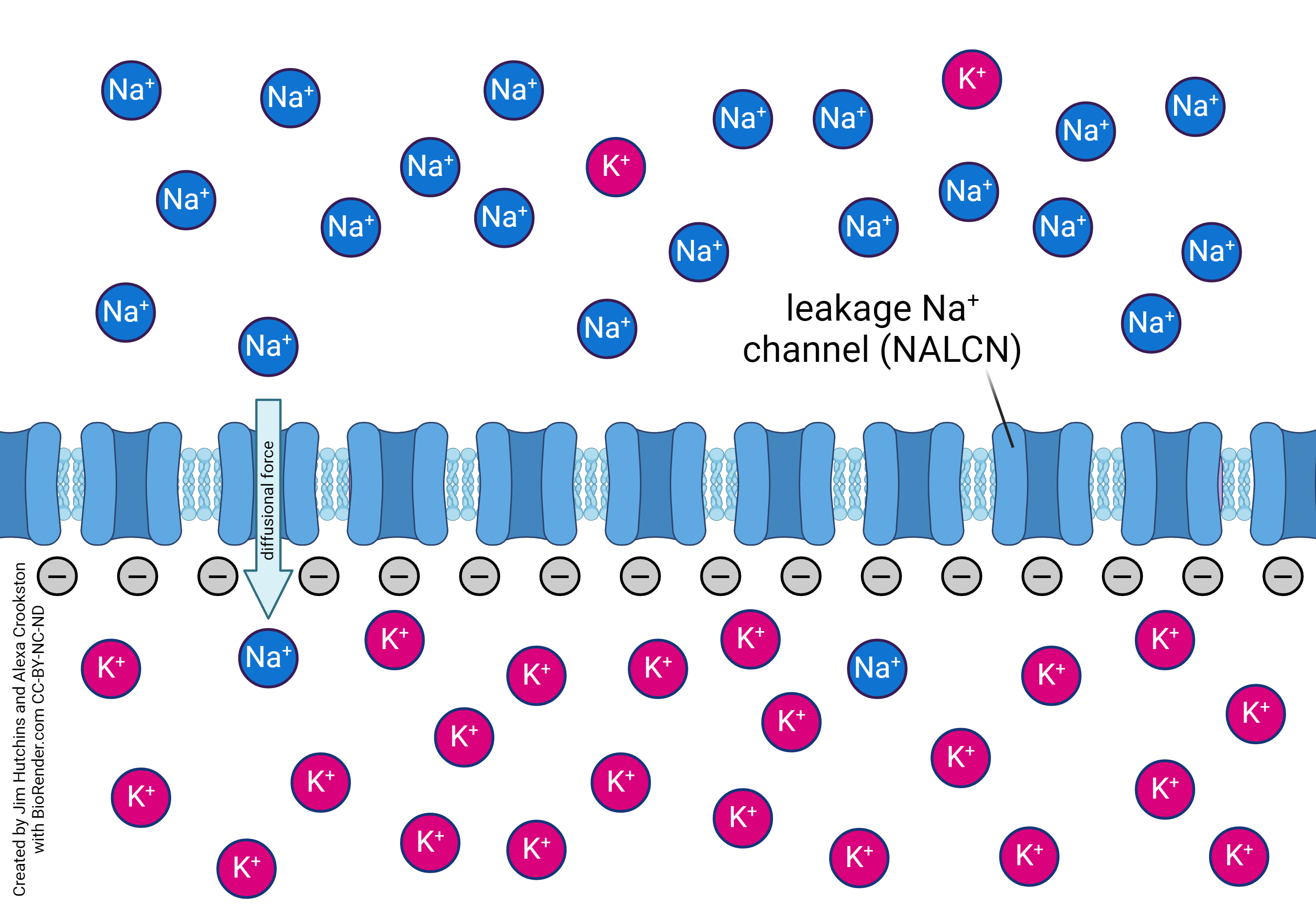
The voltage across the cell membrane when the neuron is not electrically active (the resting potential) is in part due to sodium ions (Na+) flowing down their concentration gradient (from outside to inside) through leakage sodium channels. The sodium-potassium pump is primarily responsible for moving sodium back out of the neuron. This pump consumes a large amount of the ATP used by the neuron.
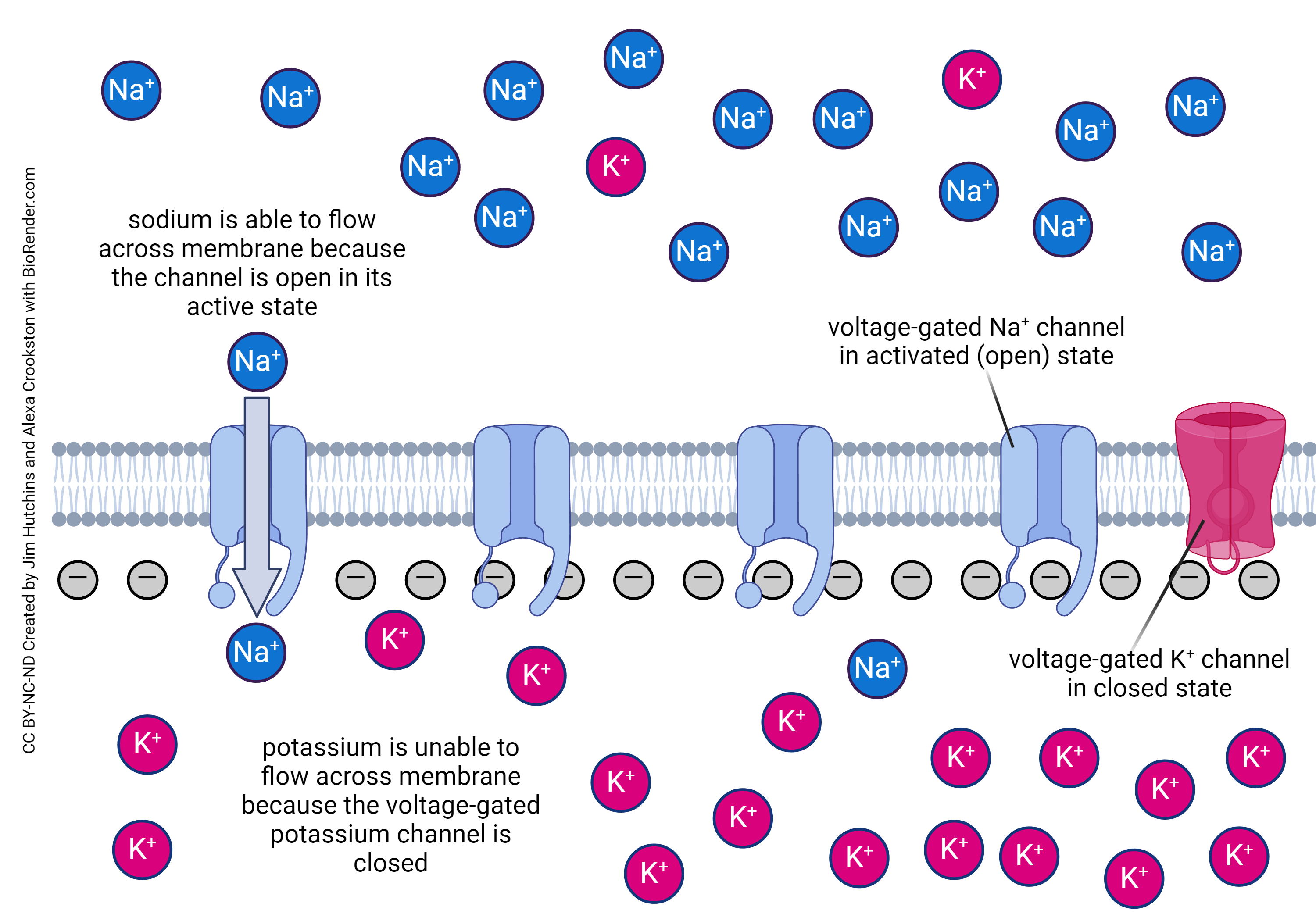
The action potential is the dynamic voltage change which neurons use as an intracellular signal that can propagate long distances, say from the big toe to the brain. During each action potential, sodium ions flow into the neuronal axon. While the axon can fire an estimated 150 action potentials based on its existing sodium gradient, with an increased number of action potentials, the sodium-potassium pump must swing into action and restore the normal sodium gradient by pumping sodium ions back out of the neuron.
Reversal of Presynaptic Ca2+
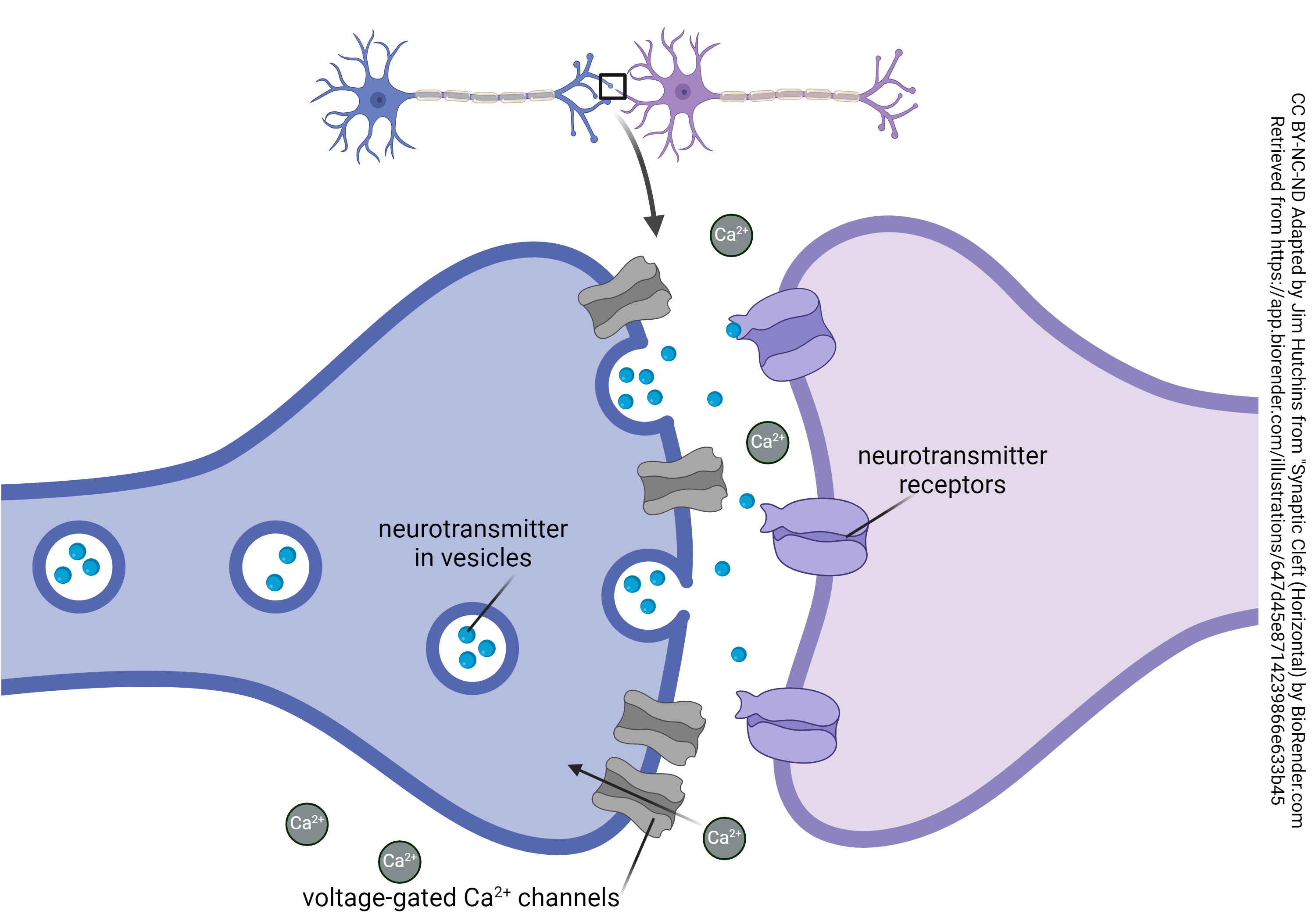
At each chemical synapse, there are voltage-gated calcium channels which (through a complex series of events) trigger the fusion of vesicles with the neuronal membrane. The calcium concentration outside the neuron is 10,000X the calcium concentration inside the neuron, and even a small amount of calcium is toxic to neurons, so it is critical that this calcium be pumped back out again by ATPases.
Glutamate Recycling
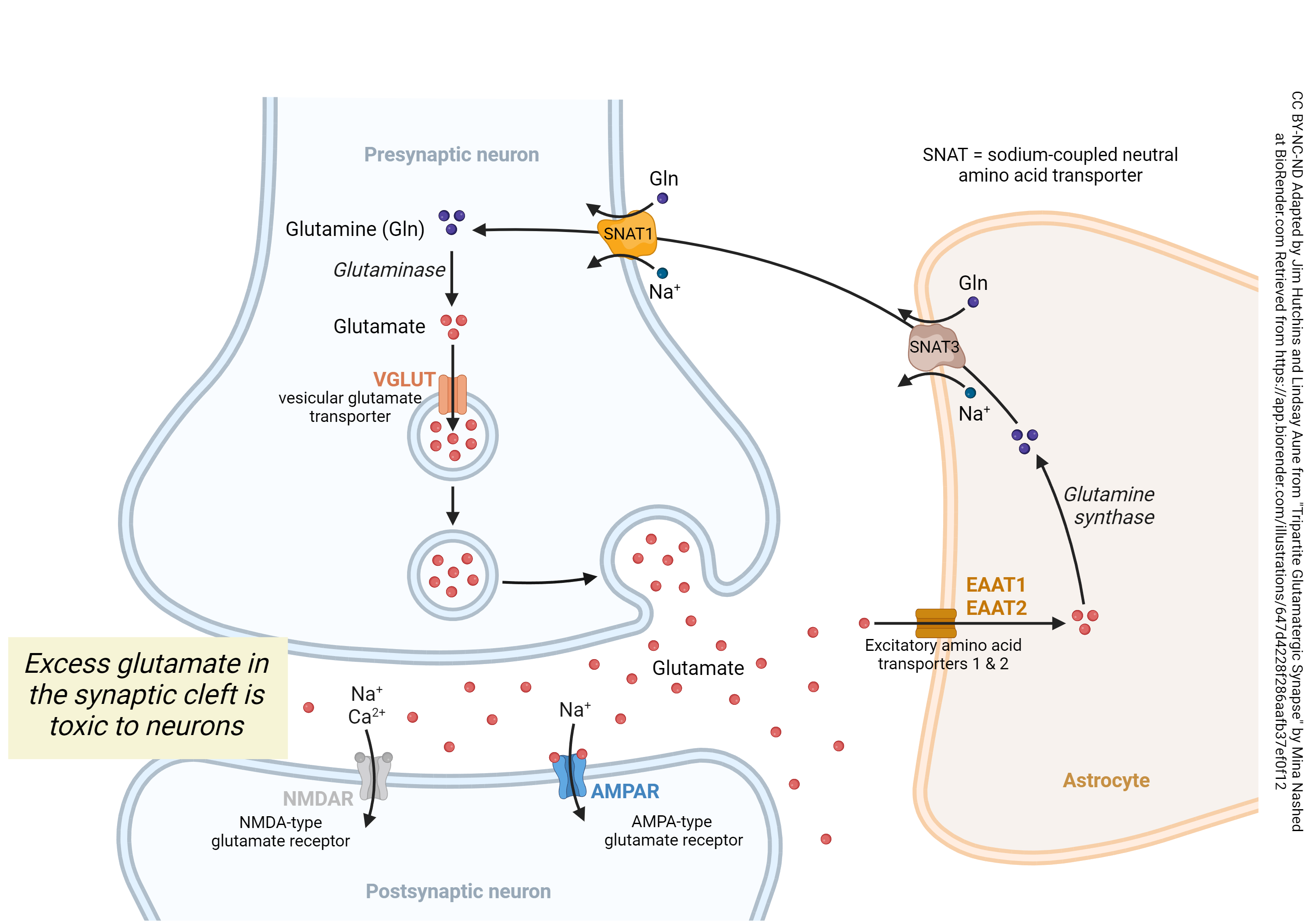
Glutamate is both the most common neurotransmitter in the brain but also incredibly toxic to neurons. To solve this apparent paradox, a significant amount of energy is used for glutamate management. Glutamate must be taken up by astrocytes, then converted to glutamine. The glutamine is at high concentration in the neuron and so is brought in by an energy-requiring symport system, a sodium-dependent neutral amino acid transporter (SNAT). The sodium ion that is brought in along with glutamine must be removed by the sodium-potassium pump.
Media Attributions
- Football and 4 pitches © Tanvir Khondokar is licensed under a CC BY (Attribution) license
- Combined Effect of Ion Channels, Pumps, and Transporters © Cierra Memphis Barnett is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Sodium-Potassium Pump © BioRender is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Vesicular Acetylcholine Transporter © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Energy usage by neurons © Rodrigo C. Vergara, Sebastián Jaramillo-Riveri, Alejandro Luarte, Cristóbal Moënne-Loccoz, Rómulo Fuentes, Andrés Couve, Pedro E. Maldonado adapted by Jim Hutchins is licensed under a CC BY (Attribution) license
- Ligand-Gated Channel © Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Sodium only diffusion only resting membrane © Jim Hutchins and Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Rising phase of action potential © Jim Hutchins and Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Voltage gated calcium channels at the synapse © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Management of Glutamate at the Synapse © Lindsey Aune and Mina Nashed adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license