Visual Transduction
Jim Hutchins
Objective 4: State the processes involved in transducing a photon into a change in neurotransmitter release in photoreceptors.
From outer to inner retina, the rod photoreceptor consists of a rod outer segment, which is a stack of membranes surrounded by the neuronal cell membrane; a rod inner segment, rich in mitochondria; a nucleus; and synaptic terminals. The membrane stacks in the rod photoreceptor are covered with a protein (opsin) complexed with an organic chemical (retinal) to form a complex called rhodopsin.
Visual Transduction in the Dark
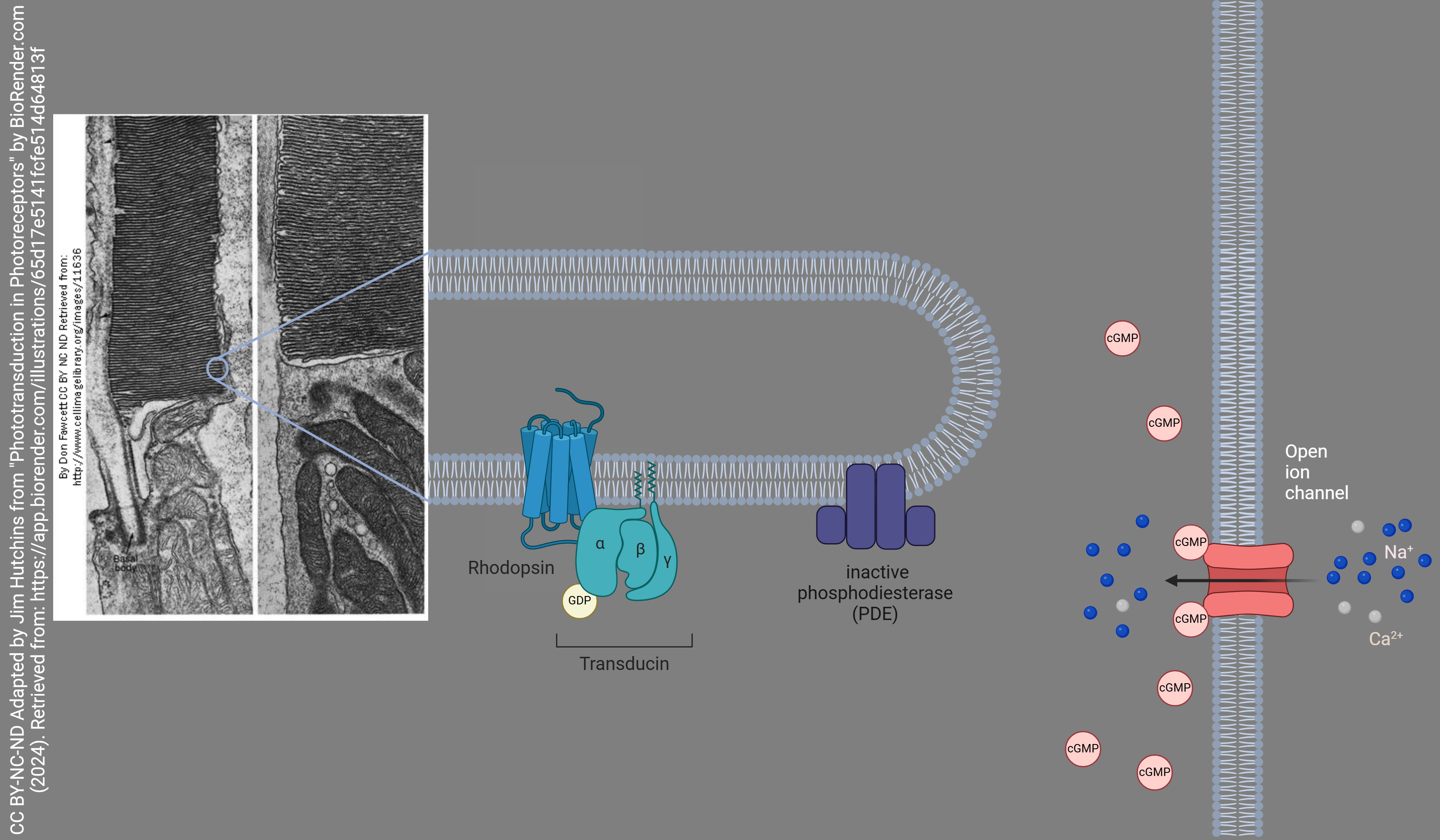
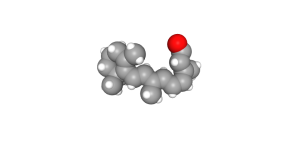
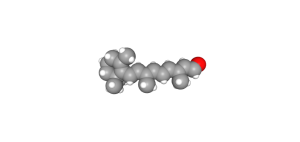
In the dark, with no photons present, retinal exists as a bent 11-cis retinal molecule. When a photon strikes an 11-cis retinal molecule, it snaps into a straight configuration called all-trans retinal. The retinal sits within the opsin molecule; when retinal changes shape, so does the surrounding opsin protein.
Opsin surrounding 11-cis retinal is associated with an inactive G protein. The inactive G protein is unable to activate a membrane-bound enzyme called phosphodiesterase. Cyclic GMP is bound to a signal-gated sodium channel in the rod cell membrane. This signal-gated channel is open when cGMP is present and sodium flows into the rod photoreceptor. This standing sodium current keeps the rod photoreceptor at a relatively high resting potential of about –40 mV. Remember from the Goldman-Hodgkin-Katz equation that a high sodium conductance will move the resting potential closer to the sodium equilibrium of about +60 mV and further away from the potassium equilibrium of about –90 mV.
Visual Transduction in the Light
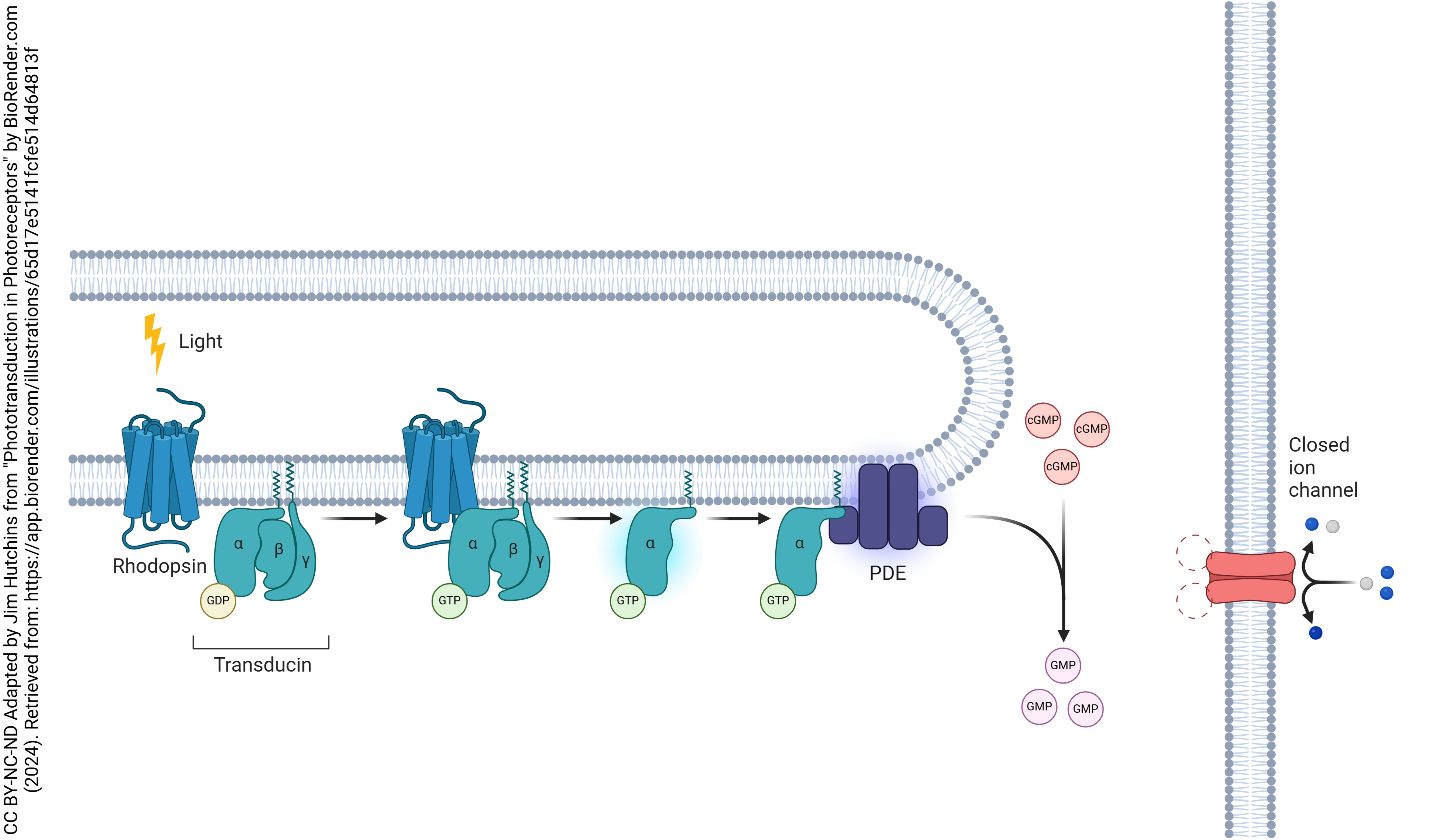
In the light, this situation changes radically. Photons strike the bent 11-cis retinal and convert it to a straight, all-trans form. This changes the shape of the surrounding opsin and the G protein becomes active.
Active G protein, in turn, activates phosphodiesterase (PDE), an enzyme which breaks down cGMP to GMP. Now cGMP no longer keeps the signal-gated sodium channel open, and it closes, which reduces the sodium conductance. According to Goldman-Hodgkin-Katz, this will move the membrane potential further from the sodium equilibrium potential and closer to the potassium equilibrium potential.
Paradoxically, in the light, the photoreceptor hyperpolarizes and less neurotransmitter is released.
When you are asleep in a dark room, the photoreceptors are depolarized, most active, and release the most neurotransmitter. As you wake and open your eyes to a new sunrise, the photoreceptors hyperpolarize, shut off, and release the least neurotransmitter.
Media Attributions
- Phototransduction in Photoreceptors Dark © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- 11-cis-retinal © PubChem is licensed under a Public Domain license
- Phototransduction in Photoreceptors Light © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- All-trans retinal © PubChem is licensed under a Public Domain license

