Skeletal Muscle
Caleb Bevan
Objective 2: Discuss the key anatomical features of skeletal (voluntary) muscle.
Function of Skeletal Muscle
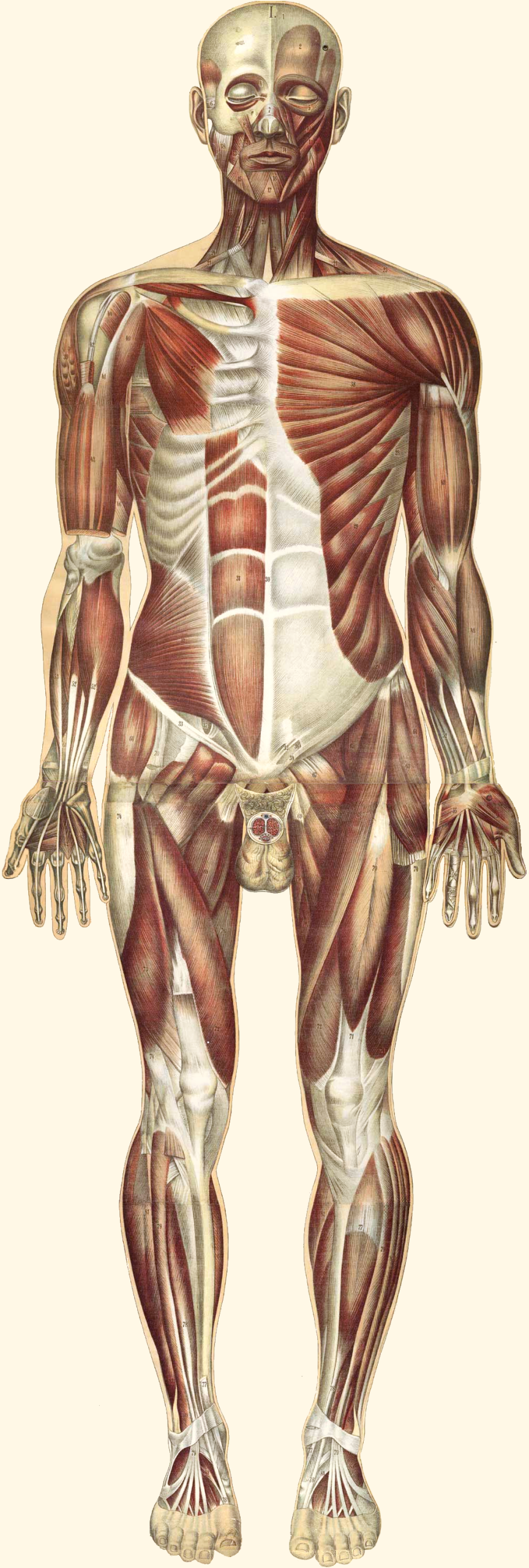
Almost half of an adult’s body weight comes from muscle, one of the four main types of tissues found in the body. Although there are three different types of muscle tissue, they all share some basic characteristics. Muscle tissue is often referred to as excitable, meaning the electrical state of the plasma membrane can change. This change, known as depolarization, occurs in response to signals from the nervous system, endocrine system or as a reaction to various intercellular signaling molecules.. Regardless of muscle tissue type, the end result of depolarization is a shortening of fibers resulting in muscle contraction.
Structure of Skeletal Muscle
Muscle cells, also known as muscle fibers, share many of the same basic structures and functions as other cells. They have nuclei, cell membranes, mitochondria, cytoskeleton, as well as the other organelles found in body cells. They perform metabolism, communicate with other cells, and are prone to cell damage and death. The most significant difference between muscle cells and other cells of the body is the density of proteins found within and surrounding the muscle cell. These proteins perform essential functions needed to allow the cell to expand and contract, which is the basis for movement in the body.
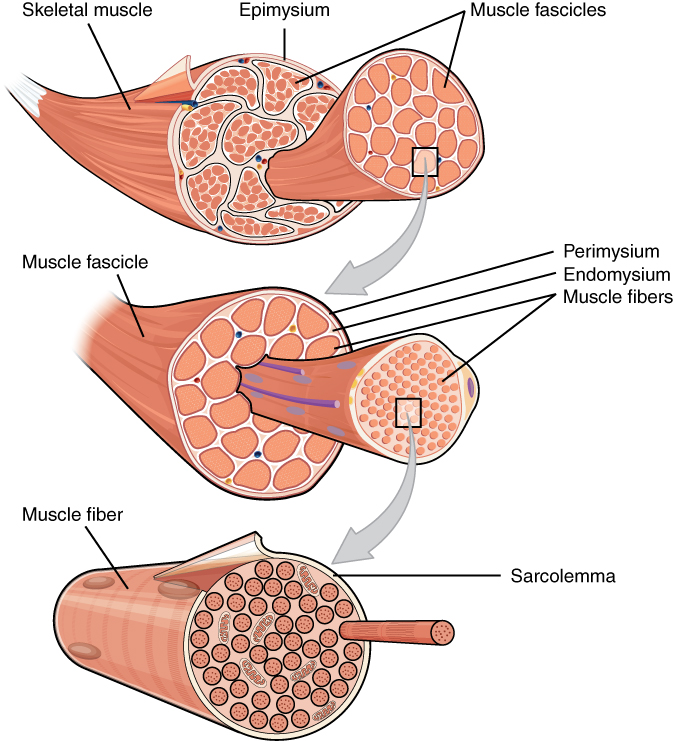
The entire muscle is surrounded by a sheath of connective tissue called the epimysium. The epimysium is continuous with a tendon, which connects the muscle to a bone. Within the muscle are a number of fascicles (Latin, “small bundle of sticks”), which give meat its visible grain. Within each fascicle are a number of muscle fibers (muscle cells), each surrounded by a cell membrane with the special name of sarcolemma. Inside the muscle fiber are a number of myofibrils which are a string of up to thousands of repeating subunits touching end-to-end.
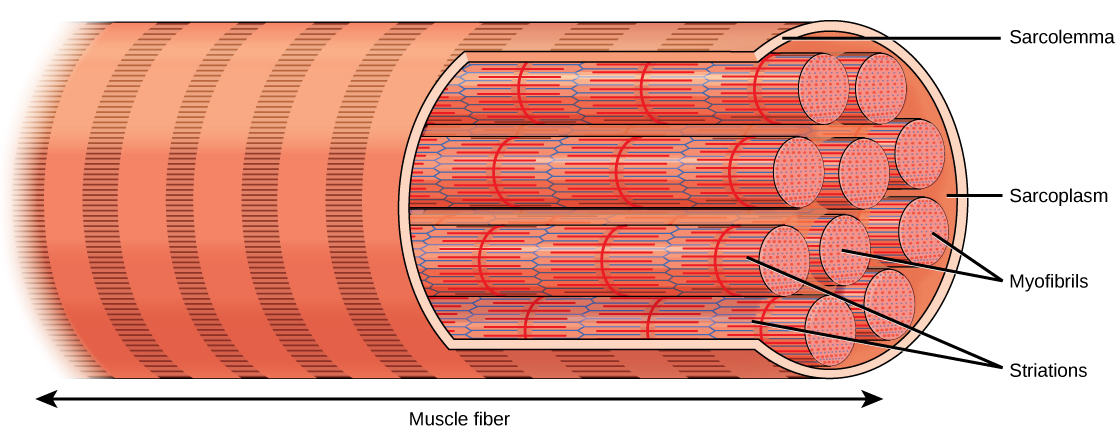
The name for one of these repeating subunits, which are the fundamental structural unit of muscle, is a sarcomere. Sarcomeres are arranged end to end in a repeating fashion throughout the muscle. This gives the appearance of striations (stripes). The sarcomere is the basic unit of muscle contraction. One sarcomere is about 2.5 μm long. When the sarcomeres collapse on themselves and become shorter, the entire muscle becomes shorter. The large rectus femoris muscle of the leg contains perhaps 150,000 sarcomeres end to end; as each of these shortens by a tiny amount, the entire muscle is contracted.
In skeletal muscle, thousands of sarcomeres form long tubes called myofibrils. These tubes are wrapped together in connective tissue and make up the bulk of the skeletal muscle cell (muscle fiber). Due to the length of each muscle fiber, many nuclei are scattered along the length of the cell. The average skeletal muscle cell is about 3 cm in length, but they can vary from about 1 mm (stapedius muscle of the middle ear) to over 50 cm (sartorius muscle of the leg).
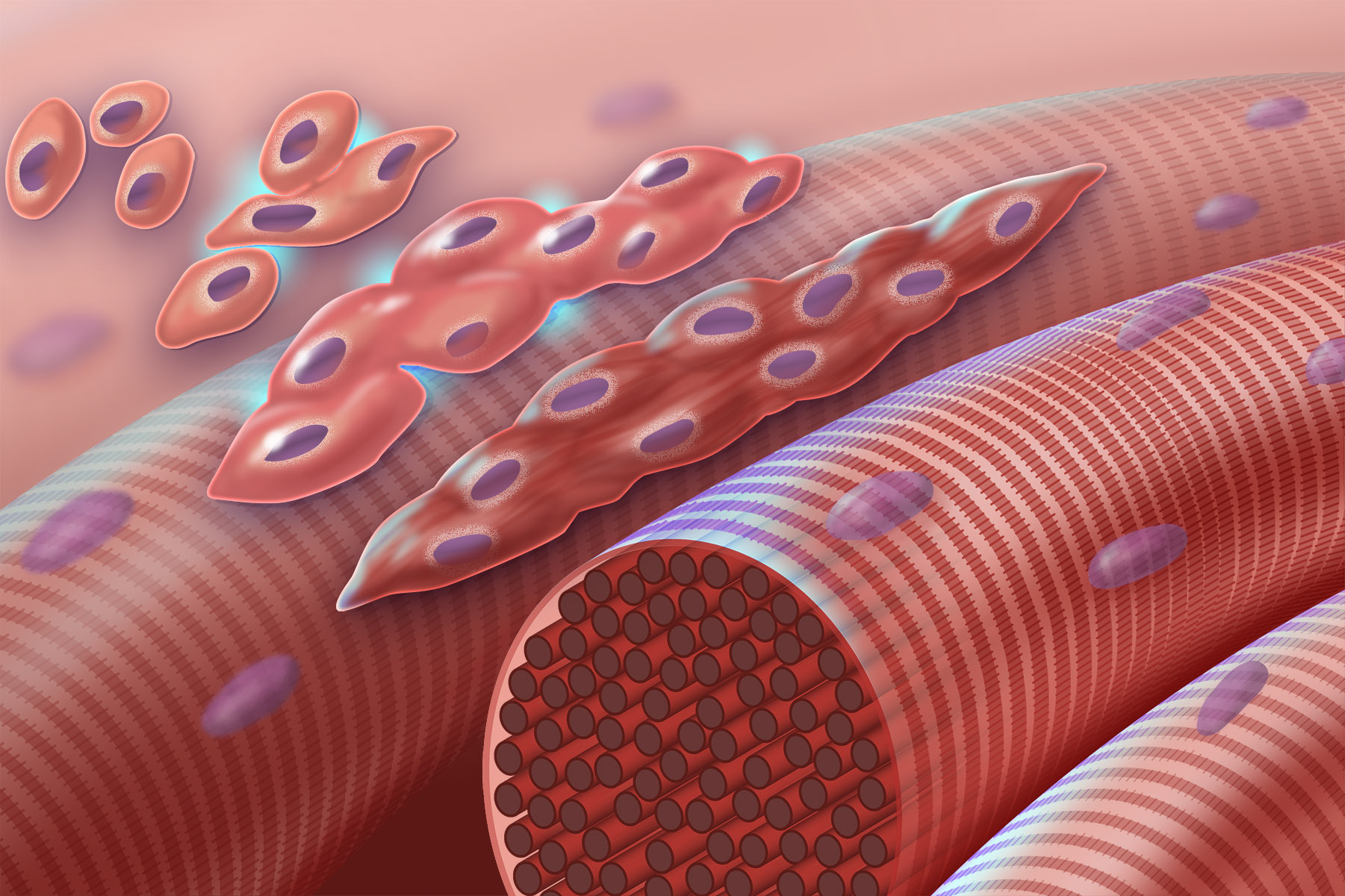 Skeletal muscle cells derive from the fusion of thousands of small cells in the embryo. This is why we see thousands of nuclei arranged along the outer perimeter of the skeletal muscle cell. For this reason, we call it a syncytium (“cells put together”).
Skeletal muscle cells derive from the fusion of thousands of small cells in the embryo. This is why we see thousands of nuclei arranged along the outer perimeter of the skeletal muscle cell. For this reason, we call it a syncytium (“cells put together”).

In the previous descriptions, you may have noticed that muscle cell terminology differs from other types of body cells.
Two Latin names appear over and over again in anatomical descriptions of muscle.
Sark or sarx (Greek σάρξ) means “flesh” in Greek, and so all the “sarco–” words come from that root: sarcoplasm, sarcolemma, sarcomere.
The Greek μῦς or mus, which became the Latin myo–, means mouse. The action of muscles like the biceps was thought to look like a mouse, probably by the same Greeks who named the constellations. They had a very active imagination.
The cell membrane of the skeletal muscle cell has the rather grand and strange name sarcolemma. It’s just like the cell membrane of any other cell; no one knows why this strange name persists.
Similarly, instead of the cytoplasm of muscle cells, we refer to the sarcoplasm.
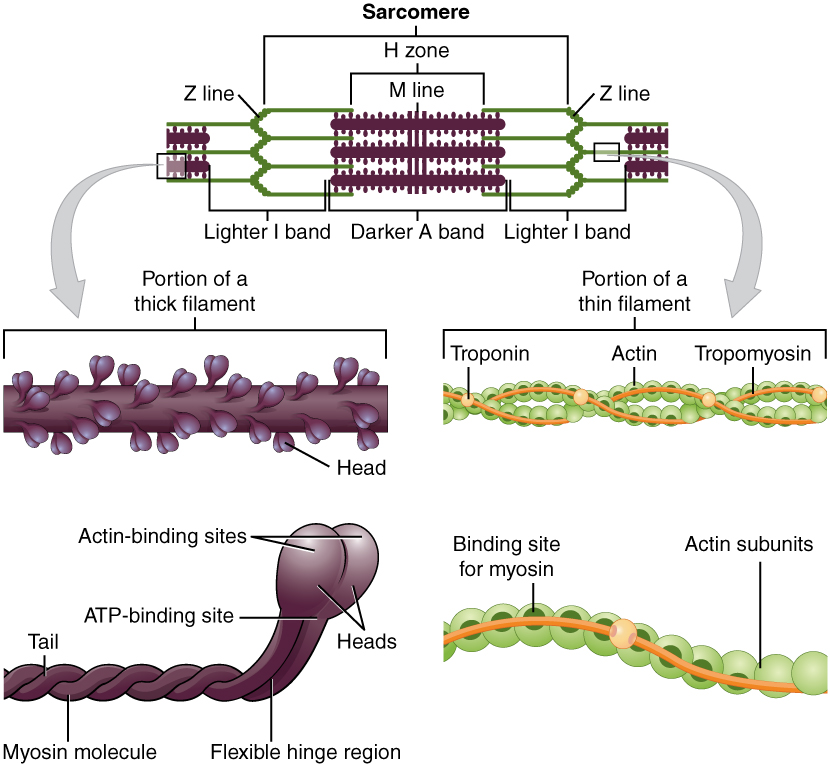
The most significant and most abundant constituents of muscle fibers are the contractile proteins actin and myosin. The interactions between myosin proteins and actin proteins result in a shortening of sarcomeres, which then ultimately shortens muscle fibers, which leads to muscle contraction. The regulatory proteins troponin and tropomyosin link the release of Ca2+ to muscle contraction. Structural proteins such as titin, actinin, and nebulin help to connect proteins within muscle fibers and maintain the shape and structure of those fibers, even during contraction. Another protein, dystrophin, serves to anchor the various proteins to the cell membrane and surrounding extracellular matrix. The protein elastin comprises the elastic fibers that are responsible for allowing muscles to stretch up to 1.5 times their resting length and recoil back to their original shape after contraction and relaxation. Many other proteins are found throughout muscles, serving to form channels across cell membranes.
Sarcomere Structure
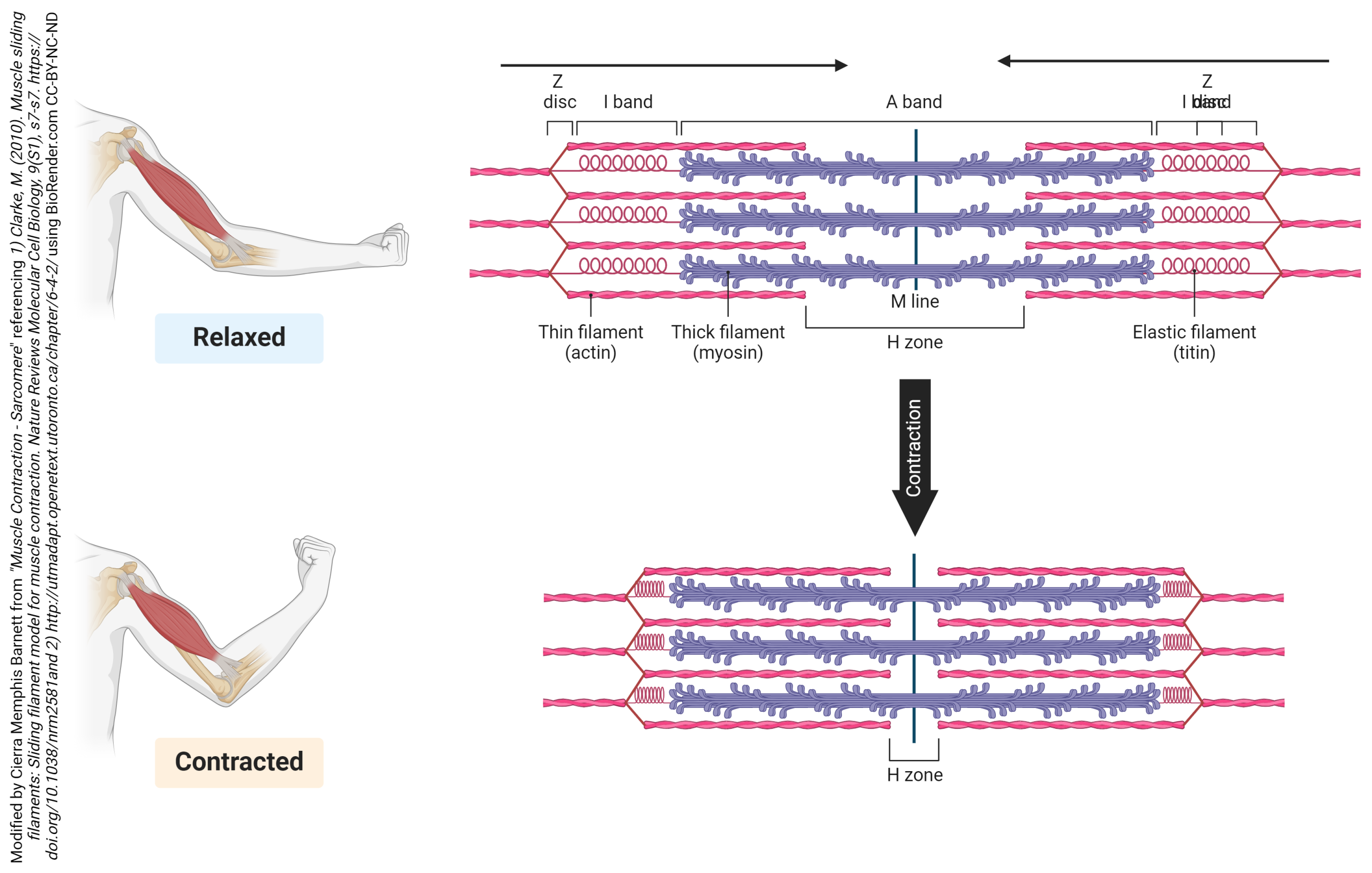 The actin filaments are held together at the Z disc (line) by a protein called actinin. The Z disc is the dark line that splits the light areas in the micrographs seen here. The Z disc defines the borders of the sarcomere. Using electron microscopy, we can see the relationship between the thick filaments of myosin and the thin filaments of actin. There are regions where only actin filaments are found near the Z discs, regions where only myosin filaments are found near the middle of the sarcomere or the M line, and regions where they overlap. These regions changes as the sarcomeres shorten or spring back to their original shape after contraction.
The actin filaments are held together at the Z disc (line) by a protein called actinin. The Z disc is the dark line that splits the light areas in the micrographs seen here. The Z disc defines the borders of the sarcomere. Using electron microscopy, we can see the relationship between the thick filaments of myosin and the thin filaments of actin. There are regions where only actin filaments are found near the Z discs, regions where only myosin filaments are found near the middle of the sarcomere or the M line, and regions where they overlap. These regions changes as the sarcomeres shorten or spring back to their original shape after contraction.
We will see in more detail later how the centrally located myosin proteins reach across the gap and bind to the actin proteins and pull them towards the center of the sarcomere. As more myosin heads bind and pull in on actin, the sarcomere continues to decrease in length. After contraction, the actin binding sites on actin are covered and structural proteins like titin and elastin help return the sarcomere to its original shape. The process is repeated every time a muscle contracts. This is referred to as the sliding filament model.
Media Attributions
- Muscle illustration © Julien Bouglé is licensed under a Public Domain license
- Muscle features © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- Fasces © Jérôme Blum adapted by Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- Muscle fiber © Clark, Mary Ann; Douglas, Matthew; Choi, Jung is licensed under a CC BY (Attribution) license
- Myoblast fusion © Darryl Leja NHGRI is licensed under a Public Domain license
- Muscle parts © Lizz Bizzell is licensed under a CC BY-SA (Attribution ShareAlike) license
- Thick and Thin Filaments © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- Sliding Filament Model © Cierra Memphis Barnett is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

