Metabotropic Receptors (G Protein-Coupled Receptors)
Caleb Bevan
Objective 5: Describe the function of a G protein-coupled receptor (GPCR).
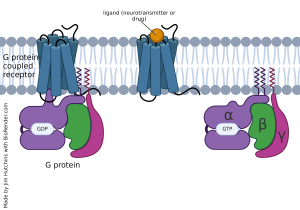 For many years, neuroscientists focused exclusively on ionotropic receptors because they were amenable to the study methods used, particularly the oscilloscope trace of voltage or current changes. Slowly, over a number of decades’ worth of research, evidence accumulated that what came to be called G protein coupled receptors (GPCRs) would represent an important class of cell surface proteins that alter the chemistry (“metabolism”) of cells when bound by ligand. GPCRs are also called metabotropic receptors for this reason.
For many years, neuroscientists focused exclusively on ionotropic receptors because they were amenable to the study methods used, particularly the oscilloscope trace of voltage or current changes. Slowly, over a number of decades’ worth of research, evidence accumulated that what came to be called G protein coupled receptors (GPCRs) would represent an important class of cell surface proteins that alter the chemistry (“metabolism”) of cells when bound by ligand. GPCRs are also called metabotropic receptors for this reason.
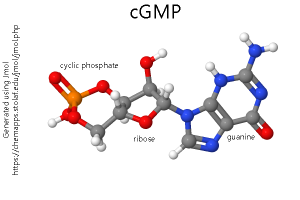
Oddly, the first inkling that GPCRs were important in the nervous system came from studies of visual transduction. (See the Visual System section for more information on this.) Here, instead of a chemical ligand, a photon is the initiator of a conformational change which ultimately results in changes in the electrical properties of the photoreceptor by changing the levels of a key biochemical called cyclic guanosine monophosphate (cyclic GMP; cGMP). Once scientists realized that most GPCRs have seven transmembrane α helices in the structure of their protein (shown in our diagrams as seven cylinders spanning the membrane), then it became much easier to identify these receptor types. In fact, we now know of many “orphan” receptors for which there is a gene sequence with seven transmembrane regions but no known ligand.
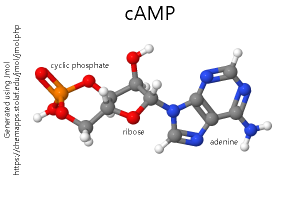
In parallel studies which did not appear at first to be related, a group of pharmacologists used a drug called forskolin to increase the activity of adenylyl cyclase (also called adenylate cyclase, or simply AC), a membrane-bound enzyme which converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cyclic AMP; cAMP). This allowed scientists to study the role of cAMP in neuronal signaling.
We now call the neurotransmitter — such as norepinephrine, dopamine, or serotonin — the first messenger. The cyclic nucleotides — cAMP or cGMP — are called the second messenger.
G protein coupled receptors are bound, as the name implies, to a complex of subunits called a G protein which is anchored to the intracellular side of the neuronal membrane. Instead of opening an ion channel, when the GPCR binds to its ligand, the shape of the GPCR changes and the inactive G protein, which was bound to GDP, becomes an active G protein bound to GTP.
G proteins associated with GPCRs consist of three subunits: alpha (α), beta (β), and gamma (γ). When the GPCR activates the G protein, the G protein often falls into pieces.
The α subunit can either activate or inhibit adenylyl cyclase. If the G protein that contains the α subunit increases the amount of cAMP made by adenylyl cyclase, then it is termed a G stimulatory (Gs) subunit. If the G protein that contains the α subunit decreases the amount of cAMP made, then it is termed a G inhibitory (Gi) subunit.
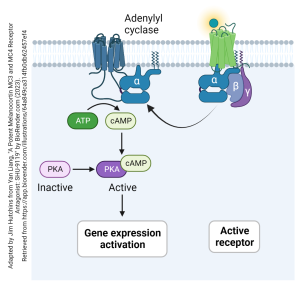
As an example, let’s look at how a Gs-type GPCR works. The ligand (shown here as a glowing ball) binds to the receptor. This activates the Gs protein and it breaks apart, releasing an αs subunit anchored to the cell membrane. This slides in the plane of the membrane, along the inside surface, until it encounters and collides with a membrane-bound adenylyl cyclase. This collision causes the adenylyl cyclase to increase its activity, and more ATP is converted to more cAMP. The cAMP second messenger can then act through a cascade to cause many different kinds of biochemical changes in the neuron. Shown here is cAMP activating protein kinase A (PKA). A kinase is an enzyme that adds phosphate groups to target proteins. This often results in changes in gene expression.
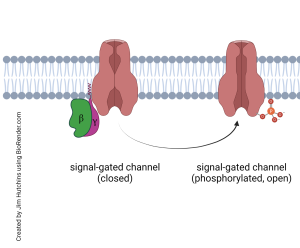
Another possible outcome of GPCR binding is the activation of a signal-gated channel. Instead of a channel that opens in response to an extracellular signaling molecule (such as a neurotransmitter), in a signal-gated channel the gate is opened by a signaling molecule on the inside of the neuron.
For example, the breakup of a G protein might release a βγ duet which slams into a signal-gated channel, causing a phosphate group to be added to it. The phosphate group then causes the signal-gated channel to be open longer or more frequently (or both), increasing the flow of ions across the cell membrane. The muscarinic acetylcholine receptor, a GPCR, acts this way. Acting through a βγ subunit pair, a signal-gated potassium ion (K+) channel is phosphorylated, causing more K+ to pass from inside the neuron to outside and hyperpolarizing the neuron so that it is now below its resting potential.
This effect is inhibitory; acetylcholine released from the vagus nerve (Loewi’s vagusstoff) onto the heart binds to muscarinic acetylcholine receptors and slows the pace of the heartbeat.
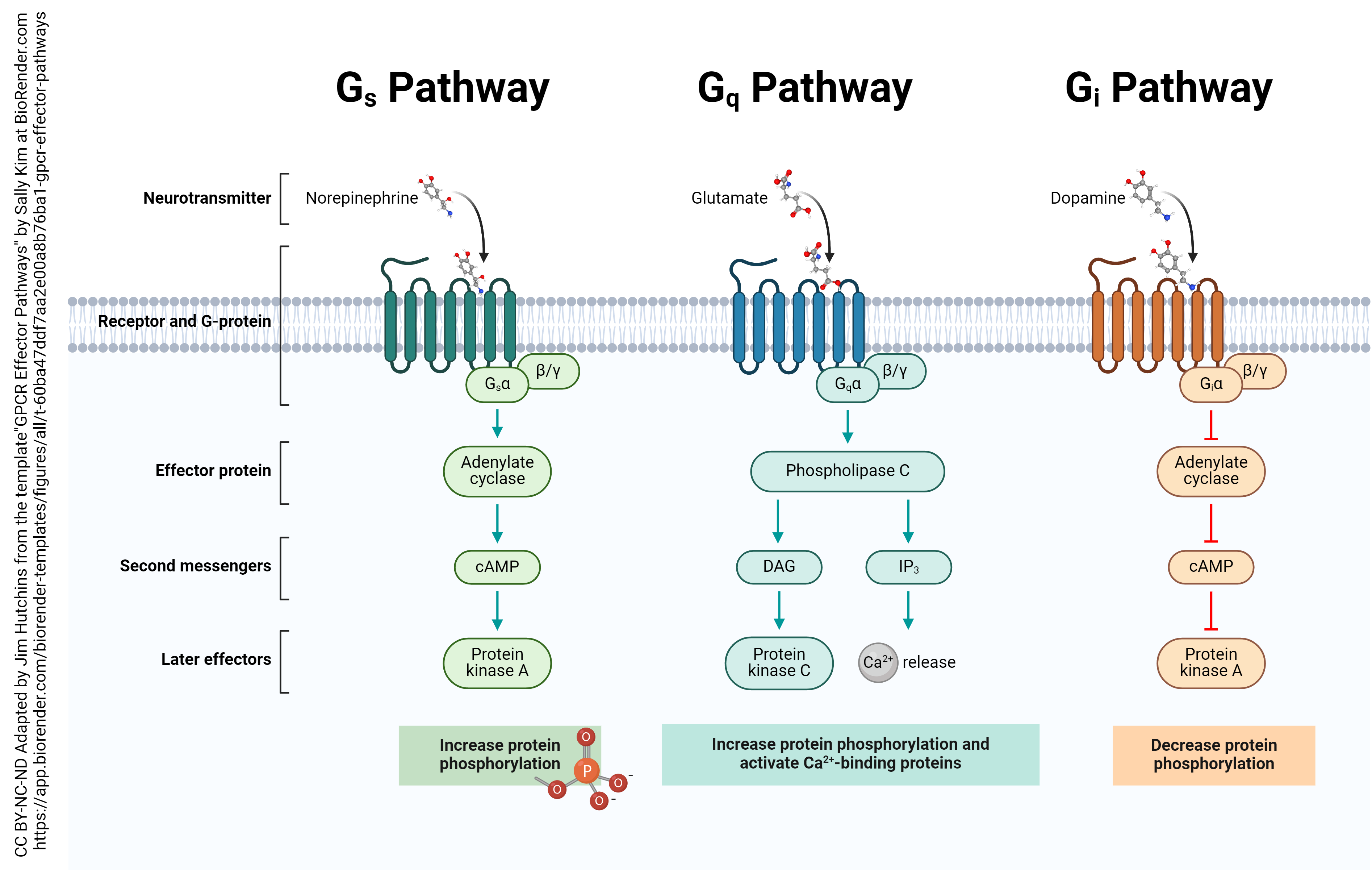
G protein coupled receptors act through a dizzying array of effectors within the neuron. We have already discussed the effects of Gs– and Gi–coupled receptors. There are also Gq–coupled receptors, that affect a molecule called inositol triphosphate (IP3) and ultimately, Ca2+ release from intracellular stores. A fourth class, termed G12/13 coupled receptors, are not at all well-understood.
Media Attributions
- G protein-coupled receptor basics © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- cGMP model © Bob Hanson is licensed under a Public Domain license
- cAMP model © Bob Hanson is licensed under a Public Domain license
- Activation of adenylate cyclase by G protein alpha subunit © Yan Liang adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Generic signal-gated channel function © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- G Protein-Coupled Receptor Effector Pathways © Sally Kim adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

