Glutamate Management
Lindsey Aune and Jim Hutchins
Objective 3: Discuss how glutamate is managed by neurons and glial cells.
Neuronal Energy Objective 3 Video Lecture
The Importance of Glutamate Management
Glutamate is the most widely distributed neurotransmitter in the human brain. About 40% of presynaptic terminals release glutamate, and about 90% of postsynaptic contacts in the brain have glutamate receptors.
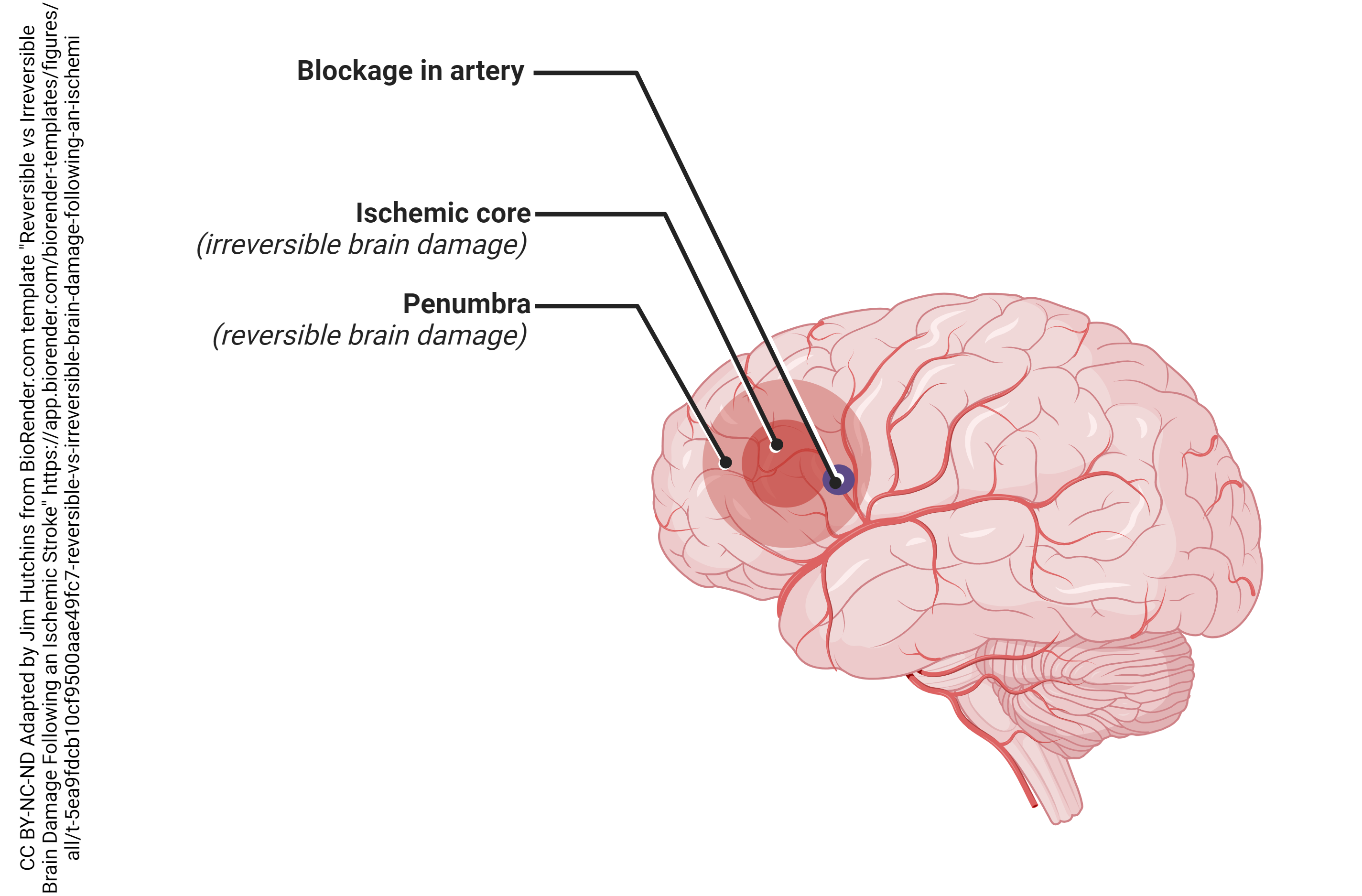 Glutamate plays many important roles in our brain. However, in large amounts glutamate can also kills neurons. If a blood vessel gets occluded, there is a loss of blood supply (ischemia) in the brain. The neurons and glial cells that are killed in the immediate area are called the ischemic core. Surrounding the core, there will be a spreading wave of damage this is called the ischemic penumbra. Because (in general) glutamate is an excitatory neurotransmitter, this phenomenon is known as excitotoxicity. As neurons die in the ischemic core, they spill glutamate into the extracellular space. This glutamate diffuses away from the site of damage and activates glutamate receptors nearby. This glutamate can cause the death of these nearby neurons, repeating the process over and over again as the area of damage spreads widely from a relatively small initial area.
Glutamate plays many important roles in our brain. However, in large amounts glutamate can also kills neurons. If a blood vessel gets occluded, there is a loss of blood supply (ischemia) in the brain. The neurons and glial cells that are killed in the immediate area are called the ischemic core. Surrounding the core, there will be a spreading wave of damage this is called the ischemic penumbra. Because (in general) glutamate is an excitatory neurotransmitter, this phenomenon is known as excitotoxicity. As neurons die in the ischemic core, they spill glutamate into the extracellular space. This glutamate diffuses away from the site of damage and activates glutamate receptors nearby. This glutamate can cause the death of these nearby neurons, repeating the process over and over again as the area of damage spreads widely from a relatively small initial area.
The Glutamatergic Synapse
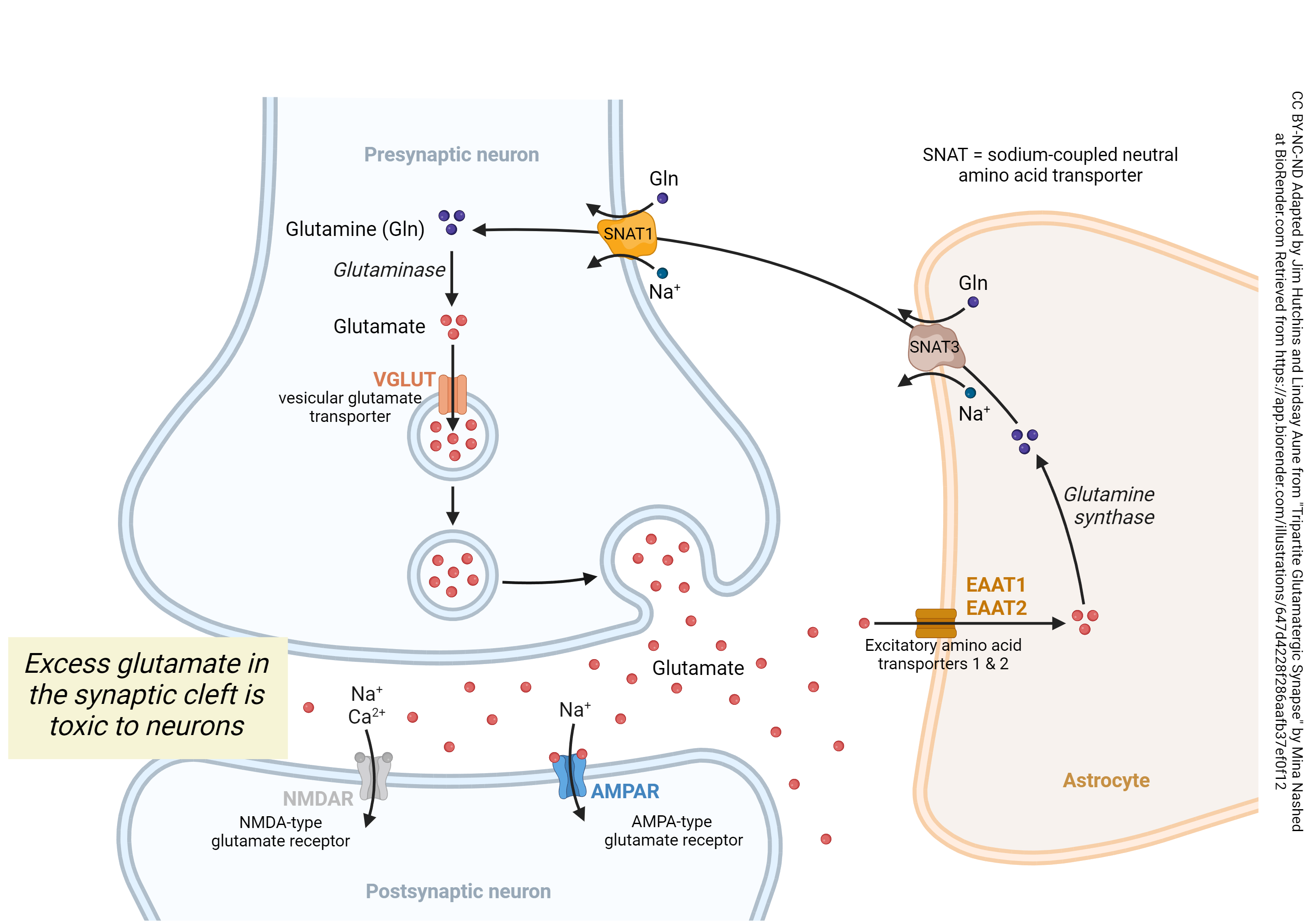
Now we’re ready to consider the entire cycle of glutamate and glutamine at the glutamatergic synapse. Glutamate released from the presynaptic neuron can activate both AMPA and NMDA receptors (AMPAR, blue; NMDAR, silver) on the postsynaptic neuron in a controlled fashion. Because excess glutamate in the synapse can become toxic for neurons, excess glutamate is taken up by the astrocyte (cell shaded brown) using the excitatory amino acid transporter 1 (EAAT1) or 2 (EAAT2).
Once safely inside the astrocyte, glutamate’s hydroxyl group is removed and replaced with an amino group to form glutamine. Because glutamine has an amino group instead of a hydroxyl group the glutamine can no longer dock at NMDA receptors (where the glutamate would previously dock)- making it non toxic to the neuron. The enzyme that accomplishes this change is glutamine synthase (also called glutamine synthetase).
Then, a transporter called the sodium-dependent neutral amino acid transporter 3 (SNAT3) carries glutamine into the extracellular space between the astrocyte and neuron. The export of glutamine is coupled to the export of Na+ which places this protein carrier in the co-transport or symport category.
Now glutamine, unable to bind to the NMDA receptor making it non toxic to the post-synaptic neuron, can be transported into the pre-synaptic neuron by a “brother” of SNAT3 known as SNAT1, also known as SLC38A1. SNAT1 is also a co-transporter carrier protein and operates in a similar fashion, bringing in glutamine and using Na+ to drive the process. (Note that in this case, Na+ is being brought into the neuron and must later be removed by the ATP-requiring sodium/potassium pump.) The mitochondrial enzyme glutaminase replaces the amino group on glutamine with a hydroxyl group, reforming glutamate once it’s safely inside the neuron.
The glutamate thus formed can be packaged into vesicles by another transporter. In this case, the vesicular glutamate transporter (VGLUT) carries in a glutamate and exchanges it for a hydrogen ion (H+). That places the VGLUT into the category of exchangers or antiport transporters. Vesicles are made acidic by a separate ATP-requiring hydrogen ion (proton, H+) pump, not shown here; in this way, H+ runs down its concentration gradient and this energy is used to bring glutamate from where it is at low concentration to where it is at high concentration inside the vesicle. This process requires energy, because it reduces entropy, and that energy is supplied by the H+ gradient (originally established by the proton pump).
Then, as explained elsewhere, glutamate can be released from vesicles at the synapse and the cycle begins again.
Media Attributions
- Ischemic Core vs Penumbra in Ischemic Stroke © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Management of Glutamate at the Synapse © Lindsey Aune and Mina Nashed adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

