Autonomic Control of Digestion
Jim Hutchins
Objective 10: Recognize the mechanisms by which the autonomic nervous system regulates digestive processes.
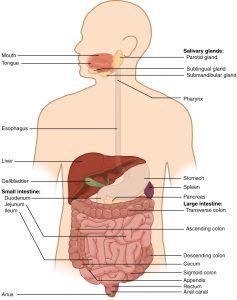
Autonomic control of digestion begins in the last 2/3 of the esophagus and continues to the internal anal sphincter.
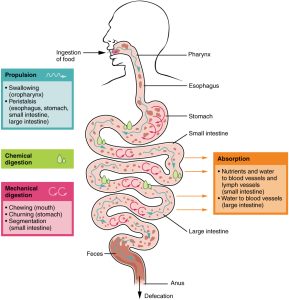
The autonomic nervous system interacts with the enteric nervous system to regulate the processes of digestion shown in this diagram. There are at least autonomic influences, and sometimes direct autonomic control, over all the processes shown here.
Myenteric and Submucosal Plexi
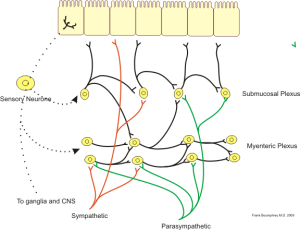
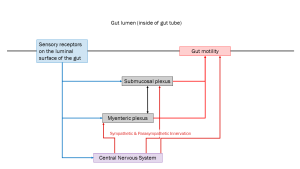
The myenteric plexus (formerly called Auerbach’s plexus), as the name implies, is mostly involved in the movement of substances in the gut. The submucosal plexus mostly controls secretion and absorption through the mucosa (cells lining the gut wall). While the activity of these neural networks is independent of other inputs, the sympathetic and parasympathetic nervous systems influence their output. In general, the sympathetic nervous system inhibits gut activity, while the parasympathetic nervous system increases gut activity.
The rhythmic activity of neurons in the myenteric plexus causes the movement of substances in the gut. This movement is called peristalsis. Acetylcholine is released onto neurons of the myenteric plexus to promote peristalsis; drugs such as atropine which block muscarinic acetylcholine receptors also block peristalsis. The neurons of the myenteric plexus control two orientations of muscle. The innermost smooth muscle fibers are oriented in a circumferential fashion (the circular layer). The outermost smooth muscle fibers run the lengthwise in the gut tube (the longitudinal layer).
Interstital Cells of Cajal (ICC)
Inside the myenteric plexus, neurons called the interstitial cells of Cajal (ICC) act as the pacemaker neurons of the plexus and set up its rhythmic activity. ICC cells are connected with each other through electrical synapses. They also make electrical synapses onto smooth muscle cells which helps coordinate their pacemaker activity. ICC cells release acetylcholine, which acts on muscarinic receptors on smooth muscle cells, as well as substance P and nitric oxide.
Peristalsis
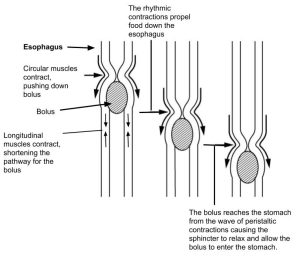
Peristalsis is a “milking” action of the muscles. Recall that a wad of food in the gut is called a bolus. The bolus is pushed forward in the gut tube by contraction of the circular muscle layers. This contraction moves forward in a wave fashion, pushing the bolus further down the gut tube as it travels. Ahead of the moving bolus, the longitudinal muscle layer contracts, which creates a smooth, tubular path for the moving bolus.
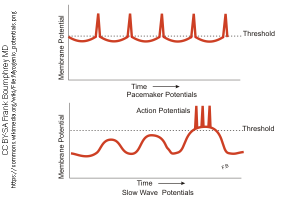
The interstitial cells of Cajal exhibit a rhythmic pattern of depolarization (top trace at left). This, in turn, causes calcium entry into the smooth muscle cells. The calcium entry is due to the opening of L-type voltage-gated calcium channels. This rhythmic Ca2+ entry eventually leads to larger and larger waves of depolarization, until threshold is reached at the crest of the largest wave and a burst of action potentials is fired (bottom trace at left).
Acetylcholine Receptors of Smooth Muscle
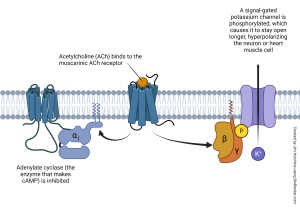
About 80% of the acetylcholine receptors in the gut wall smooth muscle cells are of the M2 muscarinic type, illustrated above. M2 receptor activation decreases the activity of adenylate cyclase and therefore lowers cAMP levels inside the smooth muscle cell. Studies with knockout mice have shown that M2 receptors are primarily responsible for the rhythmic activity of smooth muscle cells.
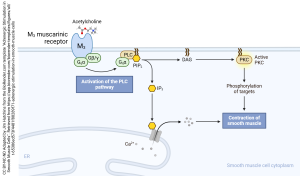
A minority, about 20%, of the acetylcholine receptors are of the M3 type, illustrated here. These are Gq coupled receptors which act through the second messenger inositol trisphosphate (IP3) to modify the release of Ca2+ from intracellular stores. Together with the acetylcholine and substance P released from the interstitial nucleus of Cajal, M3 muscarinic receptors modulate the rhythmic properties of the smooth muscle cells of the gut.
Media Attributions
- Digestive System © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- Digestive Processes © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- Enteric nervous system wiring diagram with neurons © Boumphreyfr is licensed under a CC BY-SA (Attribution ShareAlike) license
- Enteric nervous system diagram © Jim Hutchins is licensed under a CC BY-SA (Attribution ShareAlike) license
- Peristalsis © Allison Calabrese is licensed under a CC BY (Attribution) license
- Myogenic potentials © Boumphreyfr is licensed under a CC BY-SA (Attribution ShareAlike) license
- Muscarinic acetylcholine receptor © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Muscarinic M3 Stimulation in Smooth Muscle Cells © BioRender adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license

