Axonal Transport and the Tracing of Connections in the Brain
Jim Hutchins
Objective 6: Explain how anterograde and retrograde axonal transport mechanisms are used to trace connections between neurons.
Neuronal Energy Objective 6 Video Lecture
Localization of Function Historically Depended on Lesion Studies
The modern term for determining what brain region is connected to what is the connectome. (A word I rather hate, but we’re stuck with it.) As described in the chapters on the History of Neuroscience, for centuries, the only way of tracing the connectome was through lesion studies.
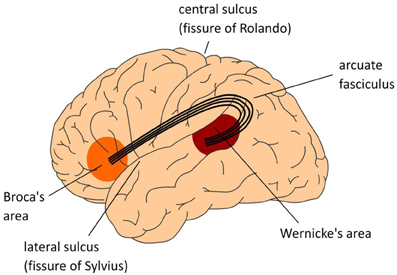 For example, Paul Broca established the location of the brain center for producing speech (Broca’s area). But a careful examination of the brains of patients who suffer strokes in Broca’s area also reveals the death of axons which end in Broca’s area, the arcuate fasciculus, and even death of cell bodies in Wernicke’s area. This is because of missing retrograde factors which keep axons and neuronal cell bodies alive.
For example, Paul Broca established the location of the brain center for producing speech (Broca’s area). But a careful examination of the brains of patients who suffer strokes in Broca’s area also reveals the death of axons which end in Broca’s area, the arcuate fasciculus, and even death of cell bodies in Wernicke’s area. This is because of missing retrograde factors which keep axons and neuronal cell bodies alive.
To make things more complicated, lots of axons in various different tracts, and lots of cell bodies, and lots of terminals, are in the vicinity of the arcuate fasciculus so there is no “pure” lesion. A whole bunch of neural structures are damaged in a stroke, or in a gunshot wound, or any other kind of brain injury.
These factors limit the usefulness of lesion studies in tracing neuronal connections.
The 1960s saw a paradigm shift. Morris Karnovsky, Marsel Mesulam, and others began exploring the use of horseradish peroxidase (HRP) as a neuronal tracer. Where HRP is active, dyes are oxidized, which may convert them from colorless to a colored, insoluble product. For example, diaminobenzidine is converted from a light brown, soluble form to a dark brown-black insoluble form which is visible in the light or electron microscope. If HRP is injected in the vicinity of axon terminals, it is taken up by the terminals and carried by retrograde transport to the cell body. This retrograde tracing method allows us to explore the origin of cells who have axons terminals in a particular region.
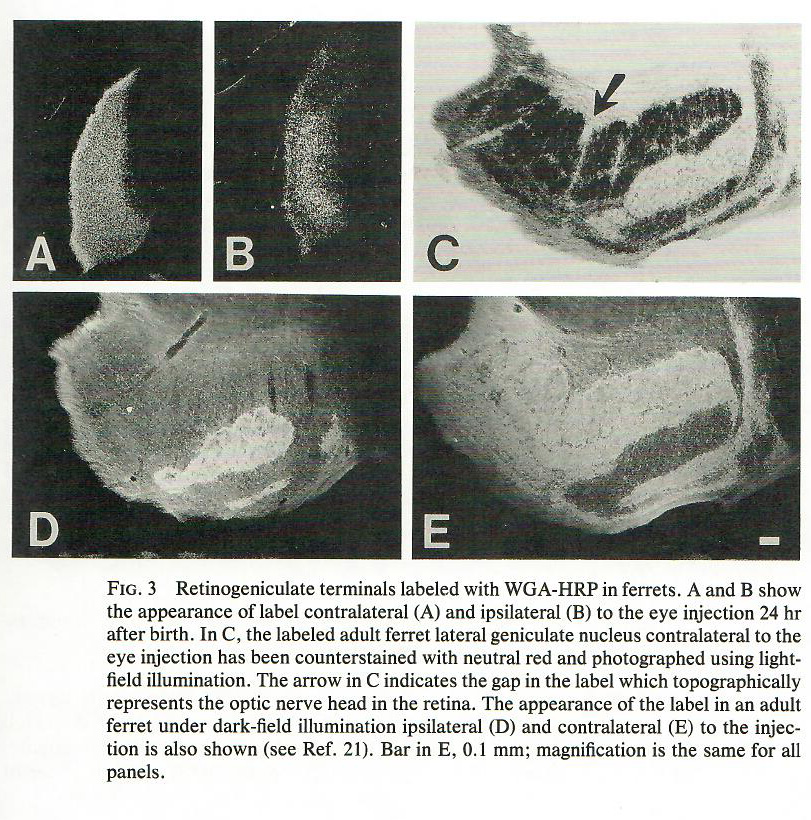
Oftentimes, however, it is more convenient to use an anterograde tracer. By conjugating wheat germ agglutinin (WGA) to HRP, the resulting larger protein (WGA-HRP) is mostly transported anterogradely, from the cell body to the terminals. For example, in this 1988 study, WGA-HRP was injected into the eye, and the terminals in the lateral geniculate nucleus (i.e. retinogeniculate axon terminals) can be visualized using diaminobenzidine.
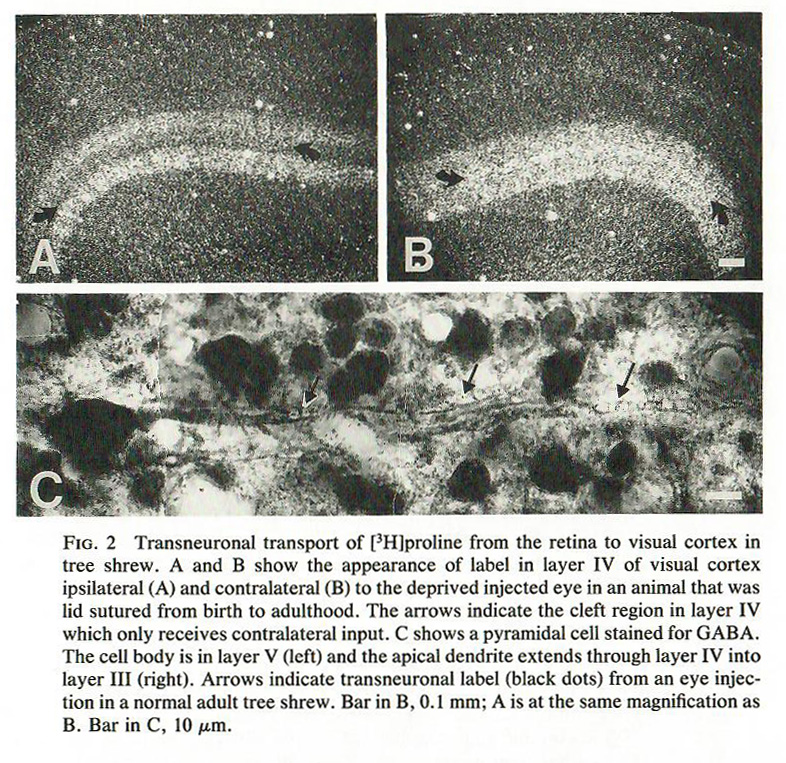
In general, neither HRP nor WGA-HRP are transported across synapses. It can sometimes be useful to know what neurons are connected through two or three synapses. For this, a transsynaptic tracer can be used. In this case, the tracer of choice in the 1980s was 3H-proline. Here, the investigators are tracing 3H-proline from the eye, through the lateral geniculate nucleus, and on to the visual cortex.
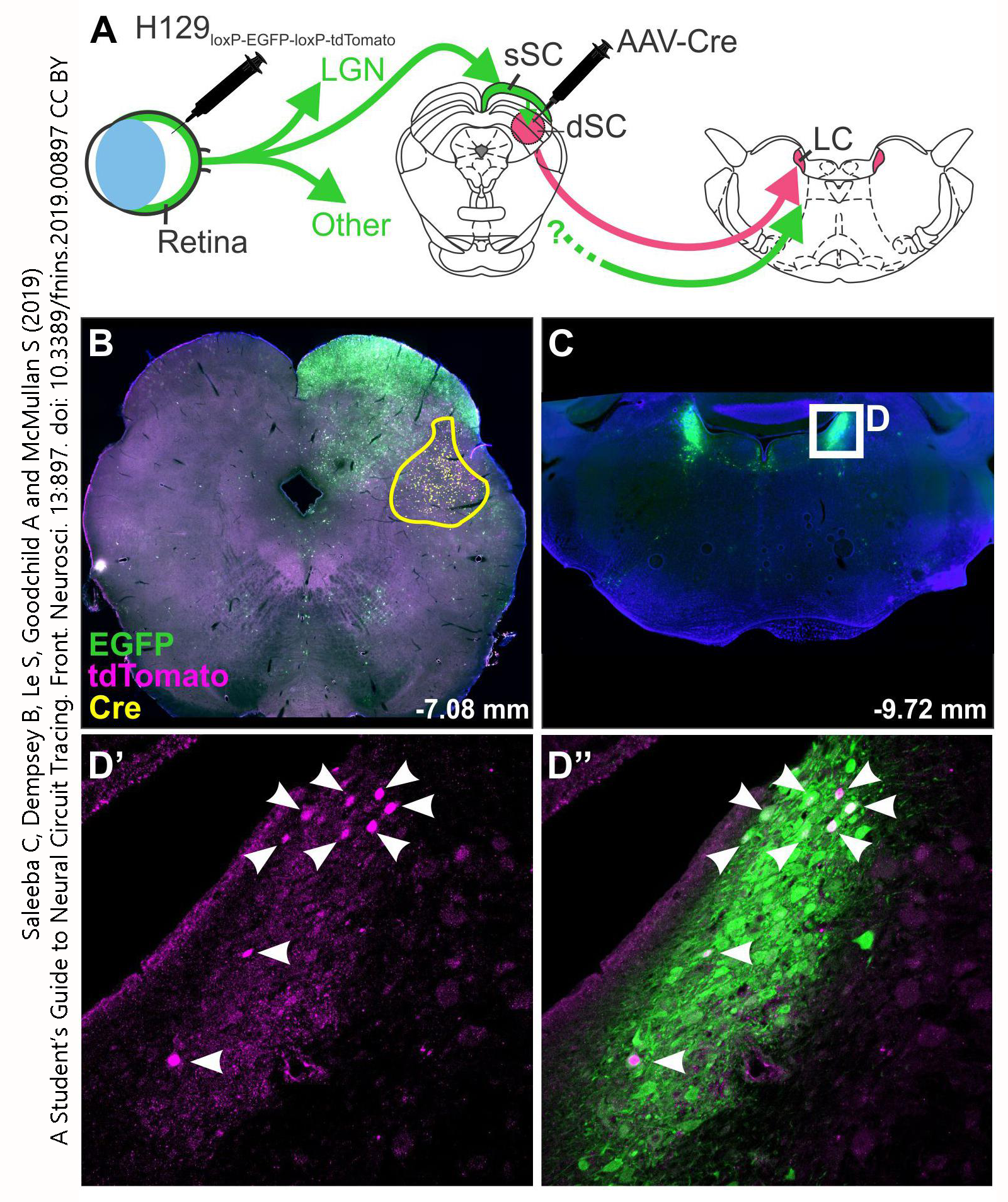
More recently, scientists have engineered viruses and proteins which are carried in one direction, and either do not travel across synapses (static), travel across only one synapse (monosynaptically restricted), or travel across multiple synapses (polysynaptic).
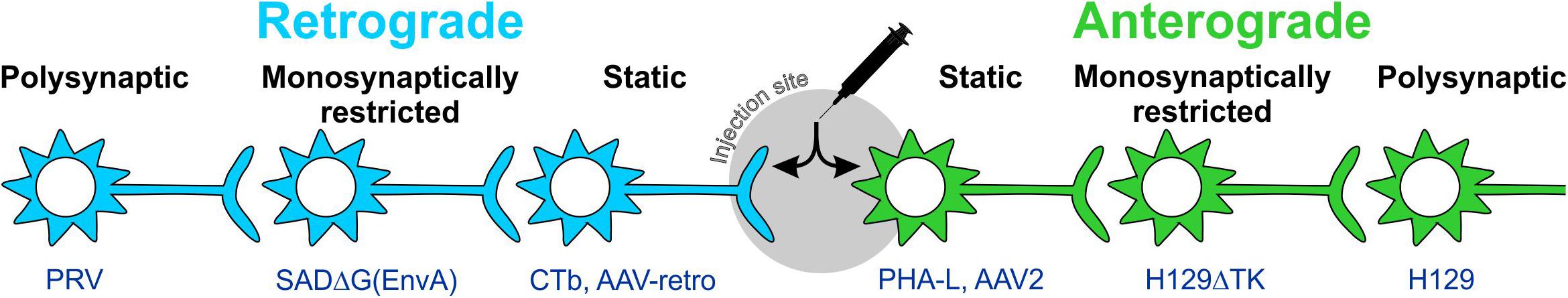
Examples of this technique are shown here. Instead of DAB, a fluorescent tag is used; this allows each virus to be “colored” differently. H129 (green) is transported anterogradely across multiple synapses. A second injection of AAV-Cre into the deep superior colliculus (dSC) activates a red fluorescent marker instead of green, so neurons which receive synapses from the retina and then project further from the superior colliculus to the locus ceruleus can be identified by their red (purple) fluorescence.
Media Attributions
- Wernicke-Lichtheim-Geschwind model of the neurobiology of language © Peter Hagoort is licensed under a CC BY (Attribution) license
- WGA-HRP tree shrew lgn development © Jim Hutchins and Vivien Casagrande is licensed under a All Rights Reserved license
- 3H-proline tree shrew © Jim Hutchins and Vivien Casagrande is licensed under a All Rights Reserved license
- Pathway tracing © Christine Saleeba, Bowen Dempsey, Sheng Le, Ann K Goodchild, and Simon McMullan is licensed under a CC BY (Attribution) license
- Anterograde transsynaptic tracing retina tectum locus © Christine Saleeba, Bowen Dempsey, Sheng Le, Ann K Goodchild, and Simon McMullan is licensed under a CC BY (Attribution) license

