Epigenetic Modifications
Elizabeth Rebarchik
Objective 5: Explain the process of epigenetic modification of genes.
Molecular Biology Objective 5 Video Lecture
Genotype
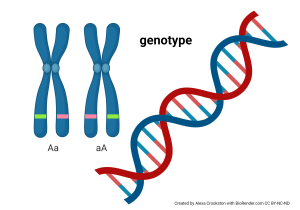 The genotype of a human is the precise sequence of 3 billion DNA bases found in human DNA.
The genotype of a human is the precise sequence of 3 billion DNA bases found in human DNA.
In the late 19th century, the Moravian monk Gregor Mendel proposed a model for the inheritance of observable characteristics. This was more than 50 years before Rosalind Franklin discovered the structure of DNA which explained inheritance at the molecular level.
Mendel proposed a genetic unit called an allele. We now know that an allele corresponds to the sequence of DNA bases on a single chromatid (i.e. a single DNA molecule).
One allele is inherited from your mother, and one from your father. Mendel proposed independent assortment of these alleles: that is, your mother has two alleles for each gene, and you have an equal chance of inheriting each one. We have already seen how those alleles are patched together in each parent by crossing over, which happens during the process of germ cell (egg or sperm) formation by meiosis.
Although it’s difficult to count the differences between humans and chimpanzees, these two species share at least 96% of their DNA. Yet a chimpanzee does not look or act 96% like a human. How can this be? Something much more important than genotype must be going on.
Still, we will use genotype as a starting point. Let’s review how the precise sequence of bases is turned into proteins, which are responsible for almost all anatomical and physiological specializations of human cells.
Epigenotype
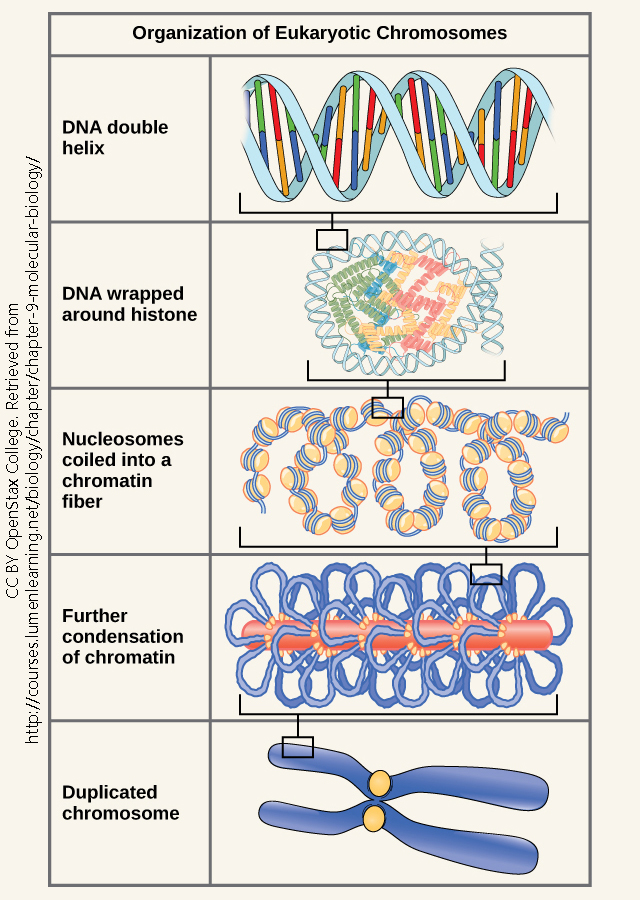
Genes can be turned “on” or “off” at different times, in different cells, by transcriptional control. Sometimes, genes are permanently turned “on” or “off” by DNA modification (methylation turns DNA “off”) or by modifying the way in which DNA interacts with histones (histone acetylation turns the nearby DNA “on” while histone methylation turns the nearby DNA “on”). Transcription factors (non-coding RNAs) can dynamically turn a specific gene “on” or “off”. All these factors make up the epigenotype of the cell.
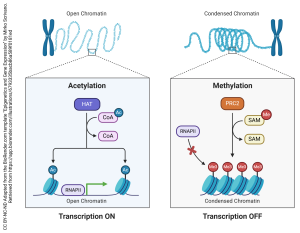
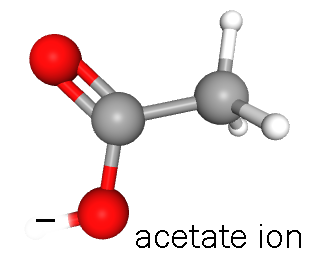 If we want to turn transcription on in a more-or-less permanent way, the enzyme histone acetyltransferase (HAT) can modify the histones by adding negatively-charged acetate groups donated by acetyl-coenzyme A. The negative charges repel the negative charges in the phosphate backbone of DNA and the structure of DNA takes on a looser, less tightly-packed configuration. This allows the enzyme RNA polymerase II (RNAPII) to access the DNA and make a primary transcript.
If we want to turn transcription on in a more-or-less permanent way, the enzyme histone acetyltransferase (HAT) can modify the histones by adding negatively-charged acetate groups donated by acetyl-coenzyme A. The negative charges repel the negative charges in the phosphate backbone of DNA and the structure of DNA takes on a looser, less tightly-packed configuration. This allows the enzyme RNA polymerase II (RNAPII) to access the DNA and make a primary transcript.
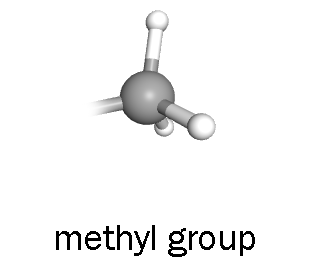 If we want to turn transcription off in a more-or-less permanent way, the enzyme polycomb-repressive complex 2 (PRC2) uses S-adenyl methionine as a methyl group to methylate histones. The non-polar groups allow DNA to bind more tightly to histones, and the structure of DNA takes on a compact, more tightly-packed configuration. This does not allow RNAPII to access the DNA and transcription cannot occur.
If we want to turn transcription off in a more-or-less permanent way, the enzyme polycomb-repressive complex 2 (PRC2) uses S-adenyl methionine as a methyl group to methylate histones. The non-polar groups allow DNA to bind more tightly to histones, and the structure of DNA takes on a compact, more tightly-packed configuration. This does not allow RNAPII to access the DNA and transcription cannot occur.
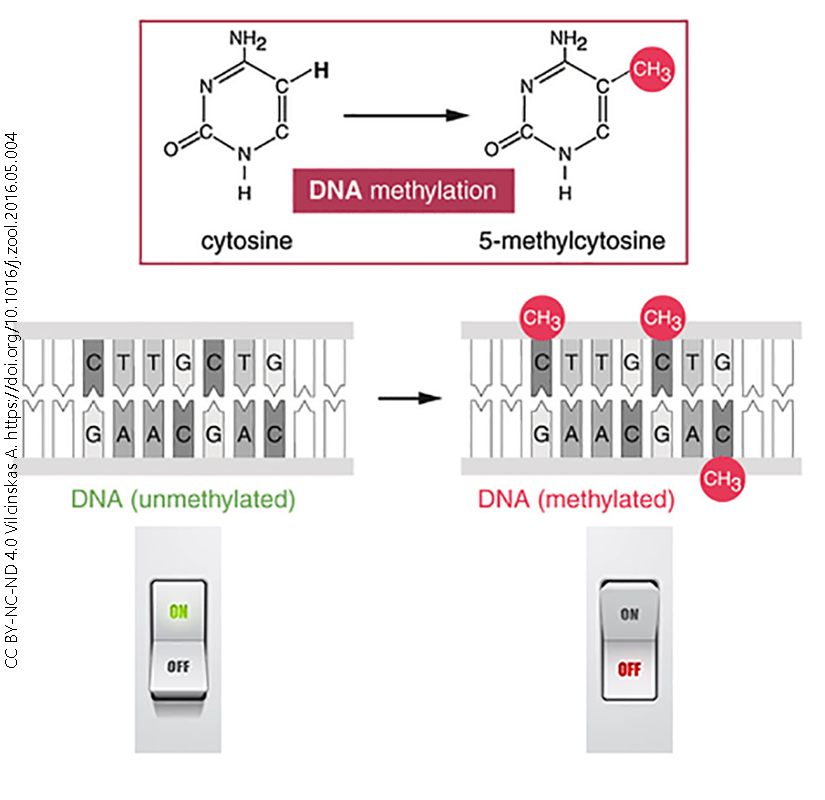 We can achieve a finer level of control over transcription by adding or removing methyl (–CH3) groups from the DNA in the gene itself, rather than the histone it wraps around. If we remove methyl groups (left), the gene is turned “on”. If we add methyl groups, they block RNA polymerase binding and the gene is turned “off”. Notice that this is a more-or-less permanent change in the structure of DNA, so these mechanisms are used when we want to permanently shut down a gene or a block of genes.
We can achieve a finer level of control over transcription by adding or removing methyl (–CH3) groups from the DNA in the gene itself, rather than the histone it wraps around. If we remove methyl groups (left), the gene is turned “on”. If we add methyl groups, they block RNA polymerase binding and the gene is turned “off”. Notice that this is a more-or-less permanent change in the structure of DNA, so these mechanisms are used when we want to permanently shut down a gene or a block of genes.
Also note that life events (stressful events, diseases, bad habits) can permanently alter the DNA and make it more or less likely that key genes will be transcribed. This can even happen in fetal life: mothers subjected to stressors while pregnant are more likely to give birth to individuals with autism, for example. Alterations in the fetal environment cause a permanent change in that child’s DNA. It is important to note that this is not in any way the mother’s fault: most, if not all, of these stressors are outside of the mother’s control.
These permanent alterations in DNA are called epigenetic modifications. Epi– means “over and above” and –genetics of course means what is coded in DNA. Epigenetics are over and above the Central Dogma of Molecular Biology.
Phenotype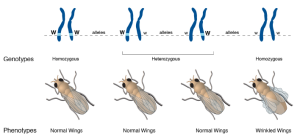
The phenotype is the collection of observable characteristics shown by an individual. The name was originally German (Phænotypus) but pheno–, as in the English word phenomenon, is from the Greek word phainein which means to “show or “shine”.
The phenotype is generally related to, but not exactly the same as, the genotype.
Take the example shown here. As Mendel suggested, there are two alleles for wing shape in fruit flies (genus Drosophila, which literally means “garbage-loving”).
Mendel called an allele dominant if inheriting only one copy caused an observable characteristic. An allele is called recessive if one has to have two copies of that allele to cause an observable characteristic.
In the case shown here, one form of the gene (allele) is designated with a capital W, while the other is designated with a lower-case w. The parents are both Ww. Each offspring has a 50% chance of inheriting a W from mother, and a 50% chance of inheriting a w from mother. Each offspring has a 50% chance of inheriting a W from father, and a 50% chance of inheriting a w from father.
Putting this together, there are four possible genotypes, each with an equal chance of occurring: they can either be WW (1/4 of the total), Ww (1/4 of the total), wW (1/4 of the total), or ww (1/4 of the total).
Because the wrinkled wings phenotype only appears if the fly inherits two lower-case w (i.e. ww), we call this characteristic recessive.
The WW genotype results in a normal winged phenotype. (Scientists call this normal appearance “wild-type” because it’s what we see in wild-caught fruit flies.)
The Ww or wW genotypes also result in a normal winged phenotype. The dominant W allele is “strong” enough to create a wild-type wing all by itself.
In the absence of the W instructions, a normal, wild-type wing cannot be made. The flies with the ww genotype are phenotypically abnormal and have wrinkled wings.
Putting it All Together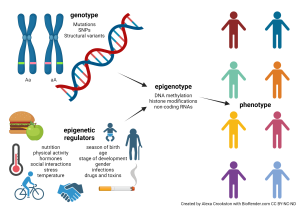
Now we can see what our intuition has told us already: very few human traits or characteristics can be explained simply by genetics. For years, evolutionary psychologists have asked of human behaviors: nature or nurture? (In other words, genes or environment?) The answer is both and neither at the same time. Rather, there is a complex and poorly understood interaction between genes and environment, acting through epigenetic mechanisms, which gives each individual a unique epigenotype. The epigenotype then is reflected in the phenotype which other individuals can observe.
Pink Flamingos
 Flamingos feathers are not genetically pink. We can see this when looking at a newborn flamingo: its genotype is to have feathers without pigmentation.
Flamingos feathers are not genetically pink. We can see this when looking at a newborn flamingo: its genotype is to have feathers without pigmentation.
The characteristic pink color of flamingos comes from a chemical called carotene, which as the name suggests is found in carrots but is also found in brine shrimp. Brine shrimp eat carotene-containing algae and the flamingos eat the algae and the brine shrimp, which turns them pink. There is an epigenetic component as well, because different birds have slightly different enzymes for metabolizing what they eat and the genes for depositing the pigment in feathers have to be turned on as well.
Race and High Blood Pressure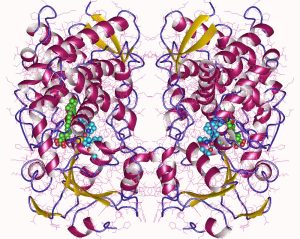
Americans of African ancestry have a higher incidence of high blood pressure (hypertension) and respond differently to antihypertensive medications than Americans of European ancestry. In fact, drug companies have marketed one antihypertensive drug, Nebivolol, for use in only African-American populations.
In the past, researchers have investigated whether this difference is genetic or environmental. Surprisingly, it’s a mixture of both. This is because both genetics and epigenetics come into play and interact with each other in unexpected ways.
Genotype plays a role. A key family of enzymes, the cytochromes P450, are involved in metabolism of different drugs, including antihypertensives. Research has shown that African-Americans tend to have a different set of single nucleotide polymorphisms (SNPs, changes in a single DNA base) in the genes for some types of cytochrome P450. We can imagine that changing amino acid –R groups in the structure of the P450 enzyme (shown at right) might have some subtle effects on its function, for example by changing the active site (“pocket”) shape. Also, a blood pressure-regulating hormone system, the renin-angiotensin-aldosterone system (RAAS), is set up differently in African-Americans than in European-Americans.
But genetics does not explain many of these differences, and diet, gender, and stress interact differently with genetics than they do in European-Americans. Male hormones regulate cytochrome P450 (subtype 4A11) gene expression. Androgens released into the bloodstream of African-American males and androgens released into the bloodstream of European-American males affect cytochromes P450 in different ways.
What matters most is not what ancestry you have (i.e. your genotype). What matters most is what ancestry you identify with. Patients who claim African ancestry do not respond to low salt diets the way patients who claim European ancestry do. Self-identified race is a better predictor of the effects of salt intake, and response to the drug Nabivolol, than genotype.
In lieu of biochemical or pharmacogenomic parameters, self-defined African ancestry seems the best available predictor of individual responses to antihypertensive drugs
It is quite likely (though certainly not proven) that the stressors unique to living as a minority in a mostly European population, such as the microaggressions, socioeconomic forces, and cultural norms experienced by self-defined Black people, interact with genetics to create the unique response of this group. Otherwise, genetic ancestry would be a better predictor than self-defined ancestry for the observed differences in phenotype.
The Sickle Cell Mutation
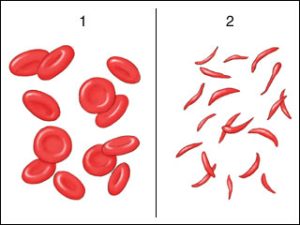
Sickle cell anemia is a genetic mutation where a single change in a single DNA base causes a change in hemoglobin, the protein which carries oxygen in the blood. In one form of the disease, a glutamic acid (GAA) codon is changed to a valine (GTA) codon. Recall from Unit 3 that the glutamic acid –R group is polar (hydrophilic) with a negative charge, while the –R group of valine is non-polar (hydrophobic). Just this single amino acid substitution results in a completely different structure for hemoglobin, from its usual “glob” shape (from which we get the name “globin”) to a rod shape. Because red blood cells are merely bags of hemoglobin, the change in the hemoglobin structure changes the red blood cell shape from round to a crescent or sickle shape.
These sickle cells tend to get stuck in capillaries, causing problems for the patients. They are also not as good at carrying oxygen as round red blood cells.
Individuals with sickle cell anemia tend to be weak and die at an early age, especially in the primordial environment. According to the theory of natural selection, anything that reduces an individual’s fitness to reproduce should eventually be eliminated from the population. Yet the sickle cell mutation persists. How could this be?
Evolutionary scientists believe there must be a countervailing benefit: the malaria parasite, which likes to live inside red blood cells, does not like to live in sickled red blood cells. Thus individuals with the sickle cell mutation are less likely to get malaria. Malaria also reduces an individual’s fitness to reproduce, and the effect of the sickle cell mutation on the ability to reproduce and pass along one’s genes, and the effect of malaria on that ability, must be about the same size.
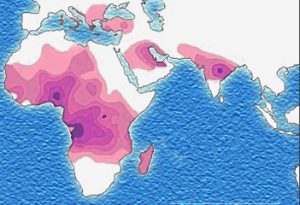

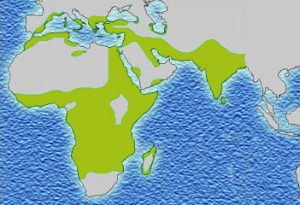
In support of this theory, the map showing the prevalence of the sickle mutation (purple) and the map showing the prevalence of malaria (green) are almost a perfect overlap.
Epigenetics and the Development of the Nervous System
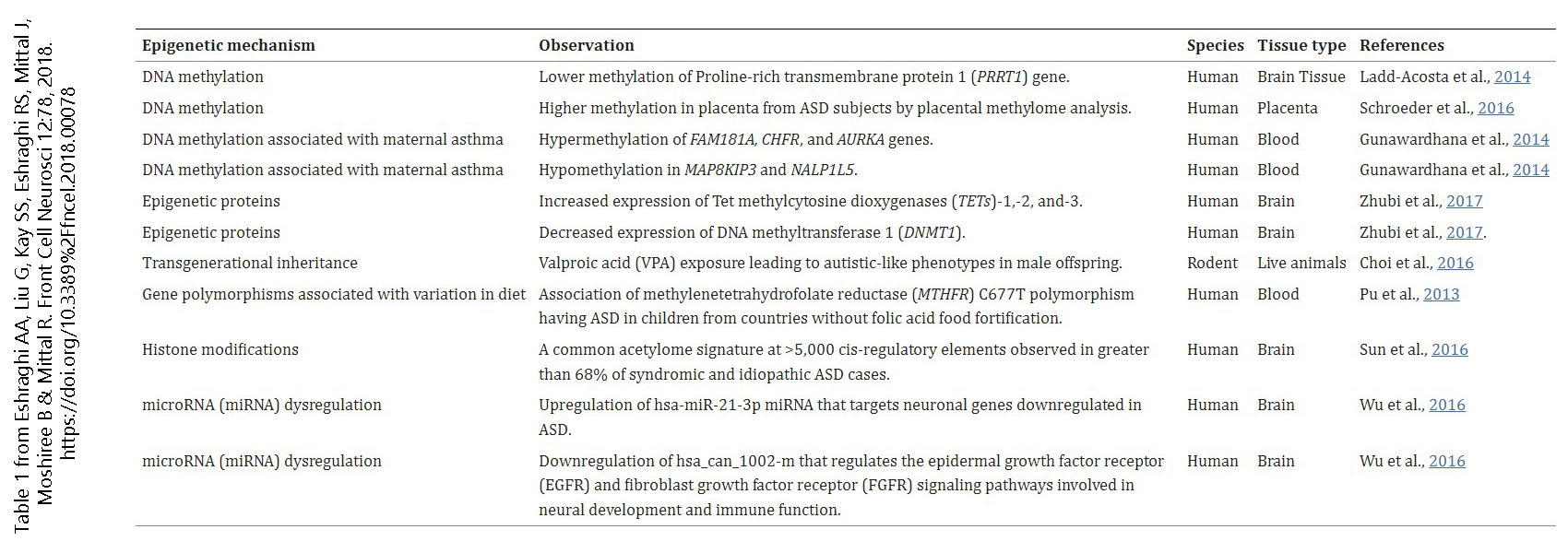
This table, from the paper “Epigenetics and Autism Spectrum Disorder: Is There a Correlation?”, lists some of the mechanisms by which epigenetic changes might lead to the alternative brain wiring known as autism.
Epigenetics video
Media Attributions
- Genotype © Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Levels of gene structure © Katherine Mattaini is licensed under a CC BY-NC (Attribution NonCommercial) license
- Epigenetics and Gene Expression © Mirco Scrivano adapted by Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Acetate © PubChem is licensed under a Public Domain license
- Methyl © PubChem is licensed under a Public Domain license
- Epigenetics methylation © Andreas Vilcinskas is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Genotype phenotype © National Human Genome Research Institute is licensed under a Public Domain license
- Genotype, Epigenetic, Phenotype © Alexa Crookston is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Flamingoes © El Golli Mohamed is licensed under a CC BY-SA (Attribution ShareAlike) license
- Cytochrome P450 © Sevrioukova, I. and Astrojan is licensed under a CC BY-SA (Attribution ShareAlike) license
- Sickle cell anemia © Pkleong is licensed under a Public Domain license
- Sickle cell distribution © Muntuwandi is licensed under a CC BY-SA (Attribution ShareAlike) license
- Mosquito © João P. Burini is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Malaria distribution © Muntuwandi is licensed under a CC BY-SA (Attribution ShareAlike) license
- Epigenetics of autism © Adrien A. Eshraghi, George Liu, Sae-In Samantha Kay, Rebecca S. Eshraghi, Jeenu Mittal, Baharak Moshiree, and Rahul Mittal is licensed under a CC BY (Attribution) license

