Energy Production
Caleb Bevan
Objective 1: Explain the way in which neurons make energy for essential cell functions.
Neuronal Energy Objective 1 Video Lecture
Adenosine Triphosphate as the Energy Currency
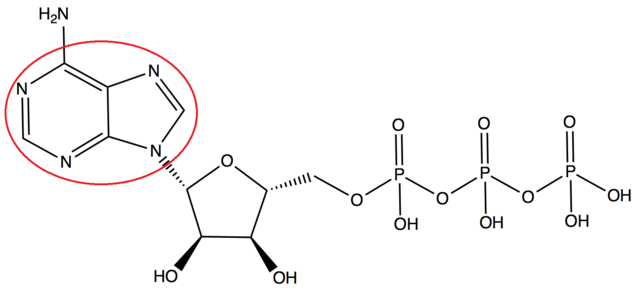
Adenosine triphosphate (ATP) is found abundantly in all body cells and is often referred to as the “energy currency” of the cell. It serves as the primary energy source for nearly all physiological processes. (For example, in climbing a flight of stairs, about 300,000,000,000,000,000,000,000 ATP molecules are consumed.)  ATP works by using the energy created by releasing energy from a phosphate group. When the third phosphate group is removed in this way, and its energy is extracted, adenosine and the remaining two phosphate groups become adenosine diphosphate (ADP). In the mitochondrion, a series of chemical reactions and a complex non-chemical process known as oxidative phosphorylation uses a chain of proteins which transport electrons to store energy in an added phosphate group as ADP is converted back to ATP.
ATP works by using the energy created by releasing energy from a phosphate group. When the third phosphate group is removed in this way, and its energy is extracted, adenosine and the remaining two phosphate groups become adenosine diphosphate (ADP). In the mitochondrion, a series of chemical reactions and a complex non-chemical process known as oxidative phosphorylation uses a chain of proteins which transport electrons to store energy in an added phosphate group as ADP is converted back to ATP.
In synaptic transmission, ATP is extremely important. It fuels the synthesis of neurotransmitters into vesicles, and enables their recycling after neurotransmitter release. ATP also helps maintain energy balance and is vital for preserving membrane potential.
ATP powers motor proteins such as kinesin and dynein, which are responsible for transporting materials along the extensive lengths of axons. It is both directly and indirectly involved in numerous essential neuronal functions.
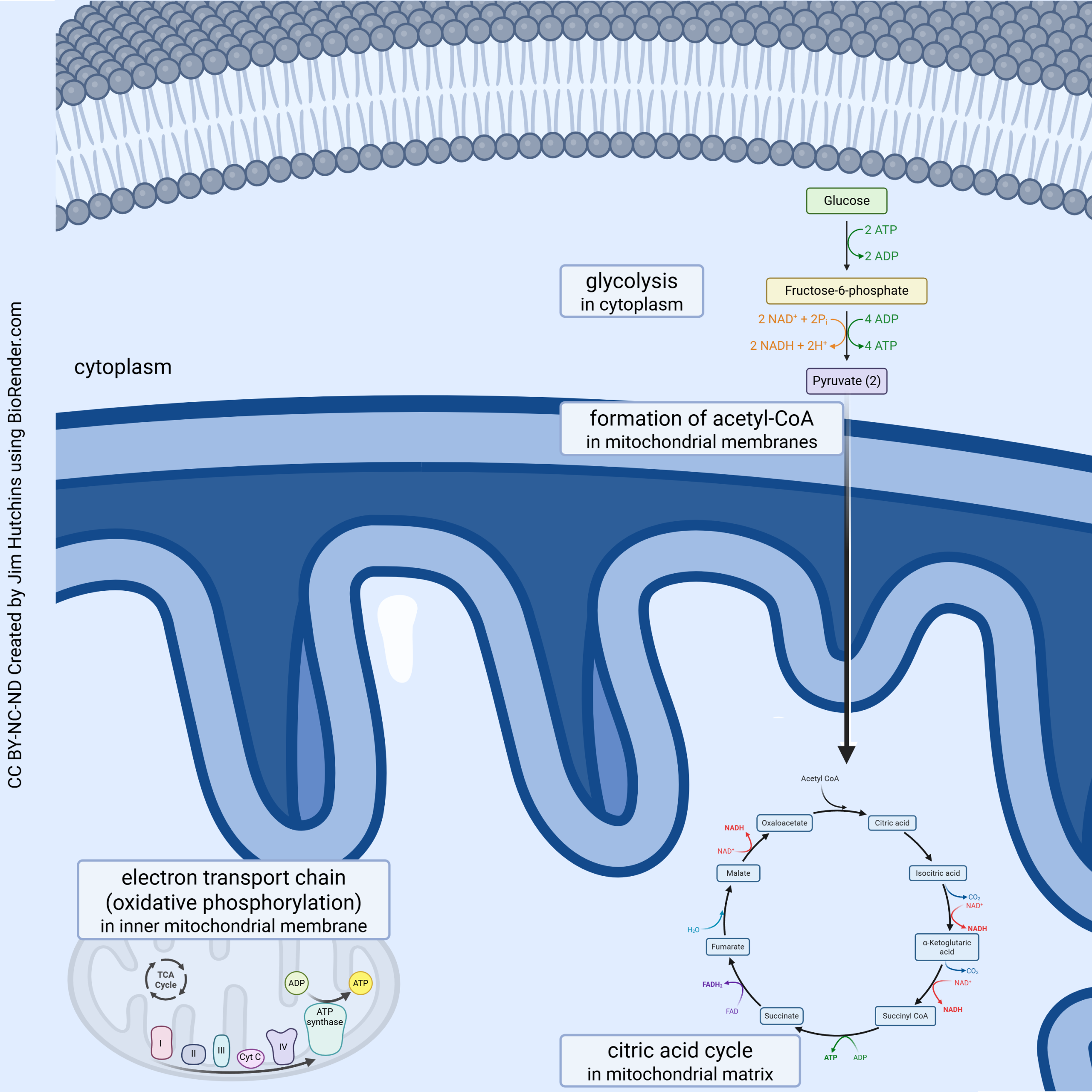 ATP is made through a four-step process:
ATP is made through a four-step process:
- In the cytoplasm, glucose is made into pyruvate (glycolysis);
- Pyruvate is carried into the mitochondrion and converted to acetyl-coenzyme A (acetyl-CoA);
- Acetyl-CoA is fed into the tricarboxylic acid (TCA) cycle;
- Electrons and protons from glycolysis, the conversion of pyruvate, and the TCA cycle are all fed into the electron transport chain.
Mitochondria: Where the Currency is Minted
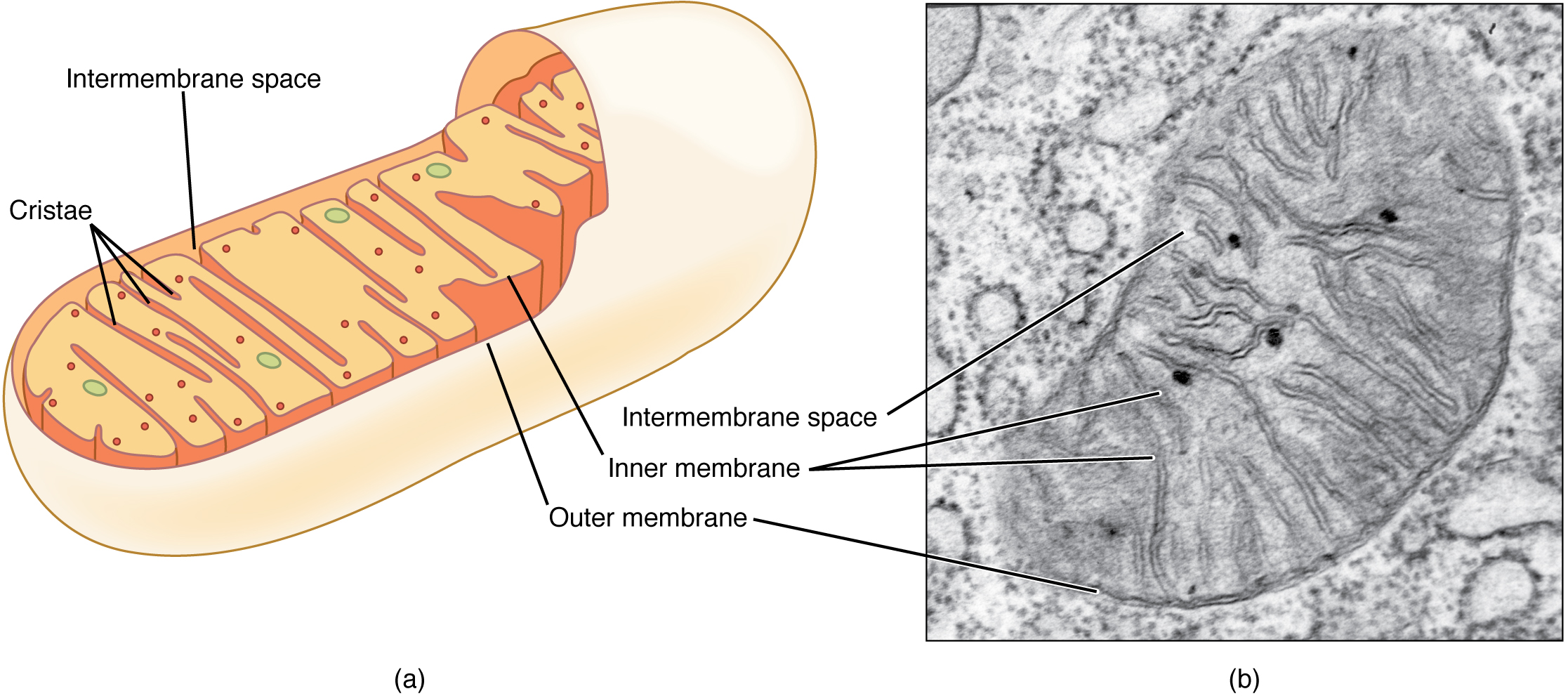
Famously, most of us are taught at an early age that “the mitochondrion is the powerhouse of the cell”. While this is true, there are many more functions of mitochondria, and this complex organelle is much more than a simple powerhouse. The mitochondrion decides which chemicals are allowed into the organelle, and is sensitive to oxygen levels. Without oxygen, the powerhouse does not take on the other half of the “fuel” needed to run the turbines, namely pyruvate. The mitochondrion houses the chemical reactions which make up the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle, after its discoverer). It also is the site of the electron transport chain (oxidative phosphorylation).
According to Lynn Margulis’ Endosymbiotic Theory, primitive mitochondria were free-living bacteria who were captured and enslaved by Archaeons about 1.5 billion years ago. This began an evolutionary line which led to the eukaryotes. Accordingly, mitochondria contain their own DNA.
Some lipids are also synthesized in mitochondria.
Glycolysis
![]()
Glycolysis is the first stage of cellular respiration. Cellular reparation is the process by which animals produce ATP in their body. When there is enough oxygen available for the reaction, glucose carried in the bloodstream enters cells and is converted to ATP. Glycolysis consists of 10 reactions that extract energy by converting a glucose molecule into a molecule known as pyruvate. Per glucose, two ATP molecules are consumed in setting up the process of glycolysis. Four ATP molecules result, so there is a net gain of two ATP molecules per glucose molecule.
If not enough oxygen is available, pyruvate cannot enter mitochondria and is converted to lacate in the cytoplasm through a process known as fermentation. Lactate (i.e. lactic acid) can accumulate in the body as a waste product. Fermentation is a familiar chemical process used to make bread and carbonated beverages such as beer.
Moving Pyruvate Into the Mitochondrion
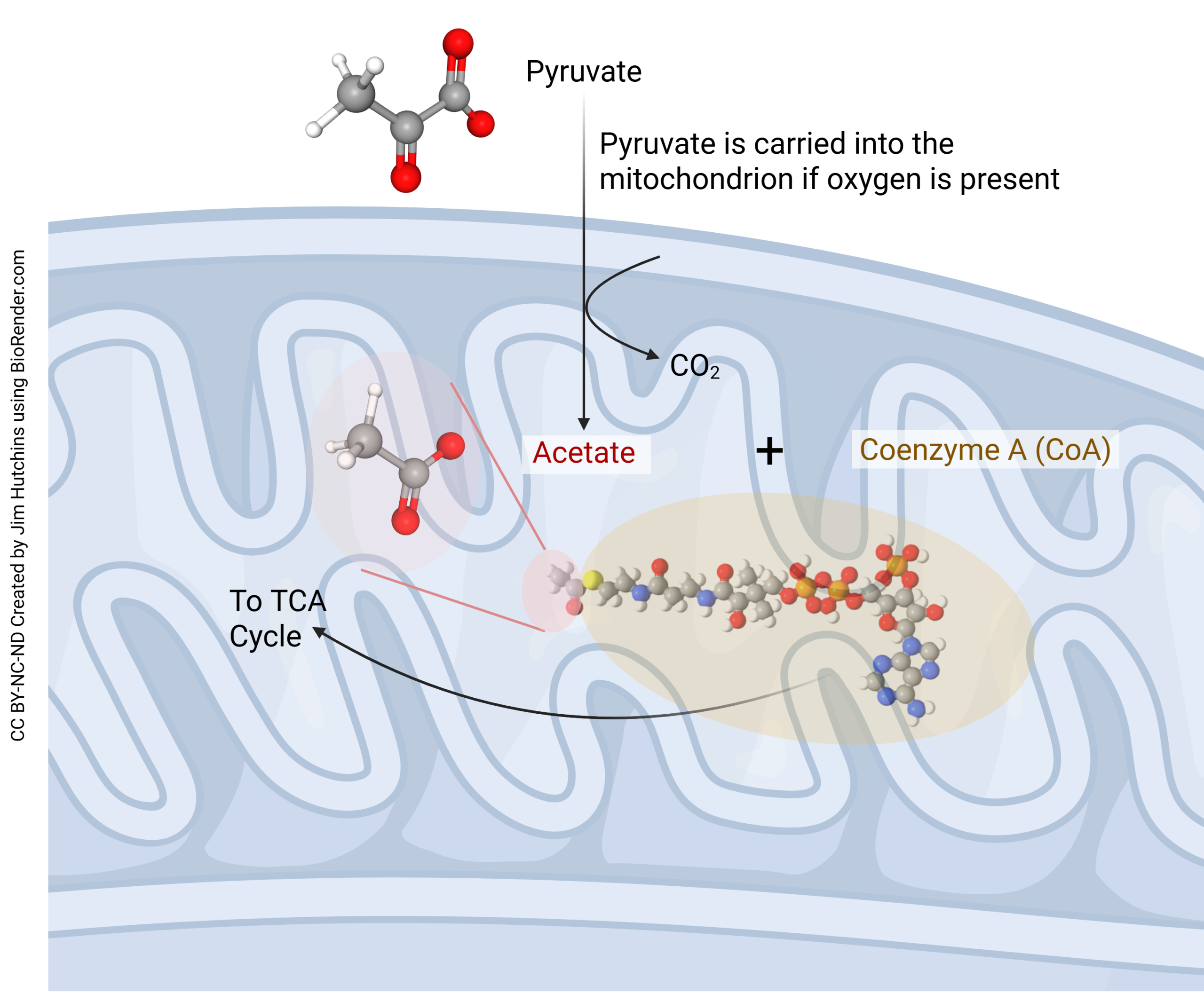 If oxygen is present, then pyruvate is carried into the mitochondrial matrix where half an O2 molecule is combined with a carbon and an oxygen from pyruvate to make carbon dioxide (CO2) plus acetate (CH3COOH). The acetate then gets picked up by coenzyme A (CoA) to form acetyl-coenzyme A (acetyl-CoA), which is fed directly into the tricarboxylic acid (TCA) cycle.
If oxygen is present, then pyruvate is carried into the mitochondrial matrix where half an O2 molecule is combined with a carbon and an oxygen from pyruvate to make carbon dioxide (CO2) plus acetate (CH3COOH). The acetate then gets picked up by coenzyme A (CoA) to form acetyl-coenzyme A (acetyl-CoA), which is fed directly into the tricarboxylic acid (TCA) cycle.
The Citric Acid Cycle (Tricarboxylic Acid Cycle; Krebs Cycle)
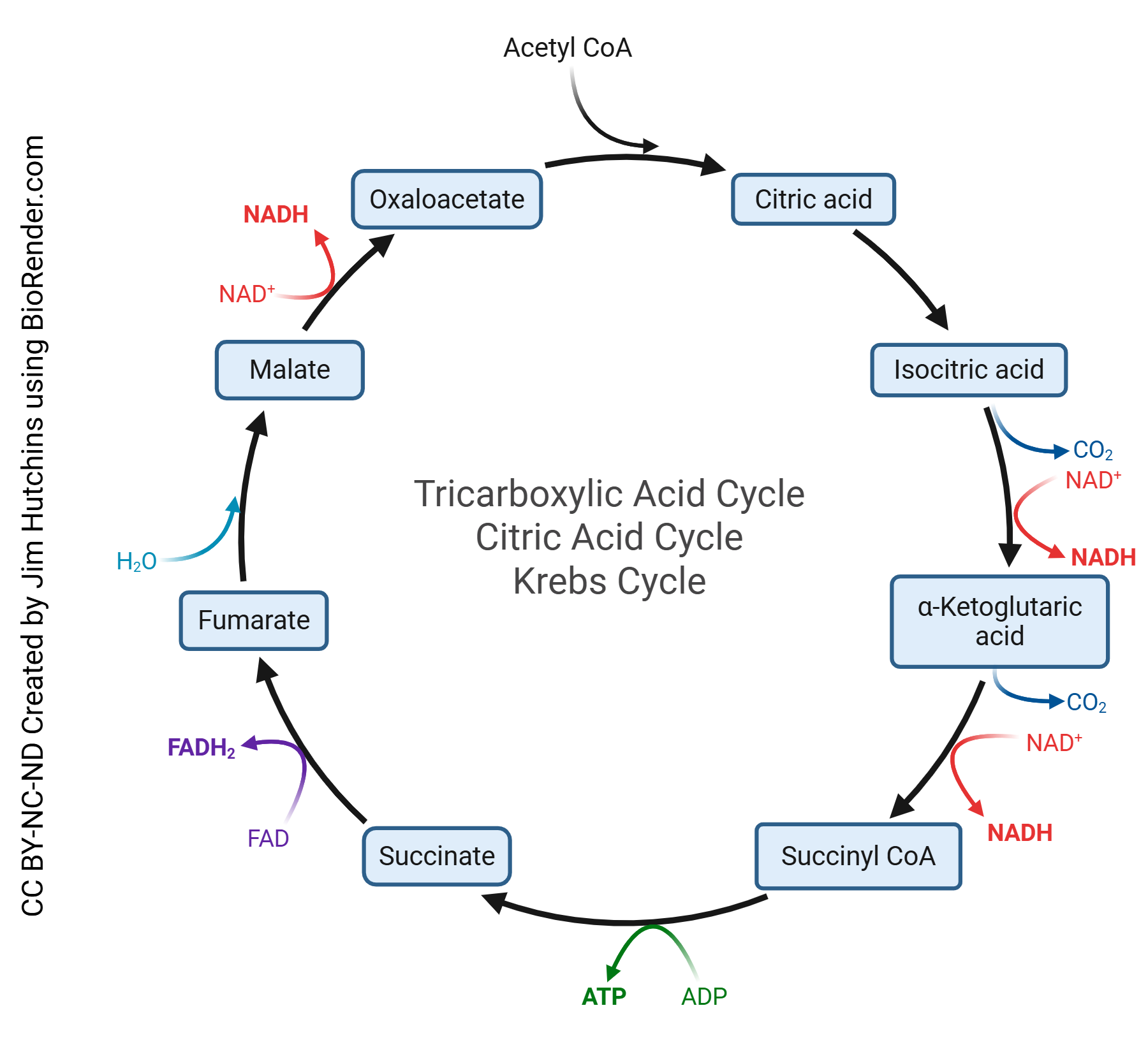
The tricarboxylic acid (TCA) cycle, also known as the citric acid cycle or Krebs cycle, is the second stage of cellular respiration and occurs within the mitochondrial matrix. The primary function of the TCA cycle is to generate electron carriers, specifically nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH₂), through a series of enzymatic reactions. The TCA cycle produces various other molecules, such as α-ketoglutarate and oxaloacetate, which serve help with other biosynthetic processes.
In the first step of the TCA cycle, acetyl-CoA is combined with oxaloacetate to yield citrate. The cycle runs going through many reactions to produce two molecules of carbon dioxide, three molecules of NADH (i.e. 3 protons and 6 electrons), one molecule of FADH2 (i.e. 2 protons and 2 electrons), and one molecule of ATP per cycle turn. One molecule of glucose make the cycle turn two separate times. The TCA cycle makes
- 4 molecules CO2
- 10 protons
- 16 electrons
- 2 molecules ATP
per glucose molecule.
The Electron Transport Chain (Oxidative Phosphorylation)
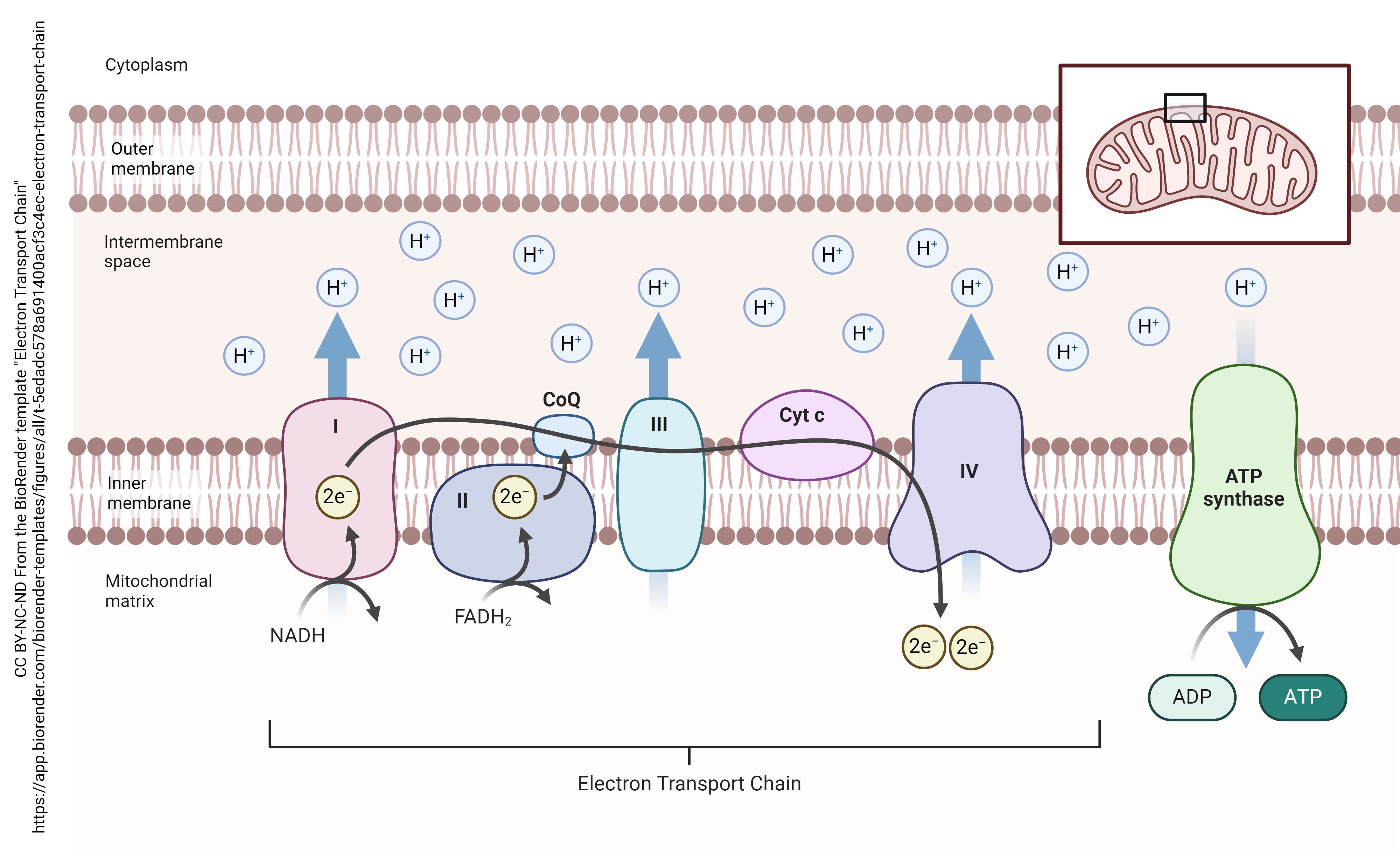
A hydrogen atom is made up of one proton (also symbolized H+) and one electron (e–). The electron transport chain sequesters the protons (H+) in the space between the inner and outer mitochondrial membrane. The electrons undergo a process called quantum tunneling where their location cannot be determined.
Glycolysis, the breakdown of pyruvate, and the TCA cycle all generate protons and electrons which are carried by FAD or NAD+ to the proteins which make up the electron transport chain. The electrons then tunnel through the series of proteins. Because they have no fixed location, they cannot be reunited with the protons trapped between the inner and outer mitochondrial membrane.
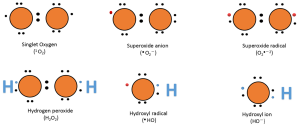
A downside of the electron transport chain is that it produces reactive oxygen species (ROS) as a byproduct. These ROS are mainly produced as a result of a premature leak of electrons from complexes I, II and III. A single electron has an ability to reduce an oxygen molecule to superoxide (O2•–). These highly reactive ROS can damage mitochondrial proteins, membranes and DNA, which could lead to oxidative stress and impairment in mitochondrial function.
To counteract these harmful effects of oxidative stress, pathways that upregulate antioxidant expression such as heat shock protein which is 70 kDa molecular weight (HSP70) are activated. HSP70 can act through a variety of ways, one of which is binding to and stabilizing proteins, to protect them against a chemical reaction with ROS. HSP70 can also help with degradation of already oxidized proteins.
At the end of the electron transport chain, the electron’s location is finally fixed and electrons are trapped inside the ATP synthase (also called the F1F0 ATPase). Now the protons (H+) may be reunited with the electrons. Before they can do that, they are forced to drive a circular track.  This track drives a rotor, which causes a conformational change in the protein. The energy generated in this way, like the rotor of a turbine, is used to recharge adenosine diphosphate (ADP) by the addition of a phosphate group and the formation of a high-energy phosphate bond, resulting in ATP.
This track drives a rotor, which causes a conformational change in the protein. The energy generated in this way, like the rotor of a turbine, is used to recharge adenosine diphosphate (ADP) by the addition of a phosphate group and the formation of a high-energy phosphate bond, resulting in ATP.
Media Attributions
- ATP adenosine © Sippit is licensed under a CC BY-SA (Attribution ShareAlike) license
- ATP space filling model © Jynto is licensed under a CC0 (Creative Commons Zero) license
- Functions of Mitochondria © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Mitochondrion anatomy © Betts, J. Gordon; Young, Kelly A.; Wise, James A.; Johnson, Eddie; Poe, Brandon; Kruse, Dean H. Korol, Oksana; Johnson, Jody E.; Womble, Mark & DeSaix, Peter is licensed under a CC BY (Attribution) license
- Glycolysis (simplified) © BioRender is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Pyruvate is converted to acetyl-CoA © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- TCA Cycle © Jim Hutchins is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Electron Transport Chain © BioRender is licensed under a CC BY-NC-ND (Attribution NonCommercial NoDerivatives) license
- Lewis dot diagrams © Mohammadi MA, Cheng Y, Aslam M, Jakada BH, Wai MH, Ye K, He X, Luo T, Ye L, Dong C, Hu B, Priyadarshani SVGN, Wang-Pruski G and Qin Y is licensed under a CC BY (Attribution) license
- Wind turbines at sea © FranceHouseHunt.com is licensed under a CC BY (Attribution) license

